Annulation
Annulation (derived from annular, occasionally annelation) in organic chemistry is a chemical reaction in which a new ring is constructed on another molecule (often another ring).[1]
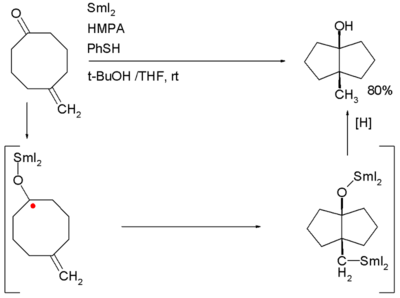
Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules are constructed from side-on condensed cyclic segments, for example helicenes and acenes. In transannulation a bicyclic molecule is created by intramolecular carbon-carbon bond formation in a large monocyclic ring. An example is the samarium(II) iodide induced ketone - alkene cyclization of 5-methylenecyclooctanone which proceeds through a ketyl intermediate:[2]
Benzannulation
The term benzannulated compounds refers to derivatives of cyclic compounds (usually aromatic) which are fused to a benzene ring. Examples are listed in the table below:
| Benzannulated derivative | Source of cyclic compound |
|---|---|
| Benzopyrene | Pyrene |
| Quinoline | Pyridine |
| Isoquinoline | |
| Chromene | Pyran |
| Isochromene | |
| Indole | Pyrrole |
| Isoindole | |
| Benzofuran | Furan |
| Isobenzofuran | |
| Benzimidazole | Imidazole |
Transannular interaction
A transannular interaction in chemistry is any chemical interaction (favorable or nonfavorable) between different non-bonding molecular groups in a large ring or macrocycle.[3] See for an example the molecule atrane.
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "annulation". IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "annelation".
- ↑ Construction of Bicyclic Ring Systems via a Transannular SmI2-Mediated Ketone-Olefin Cyclization StrategyGary A. Molander, Barbara Czakó, and Michael Rheam J. Org. Chem.; 2007; 72(5) pp 1755 - 1764; (Article) doi:10.1021/jo062292d
- ↑ Experimental evidence in support of transannular interactions in diketones Kata Mlinaric-Majerski, Marijana Vinkovic, Danko Škare, Alan P. Marchand Arkivoc DS-339E 2002 Online Article