Tobacco mosaic virus
| Tobacco mosaic virus | |
|---|---|
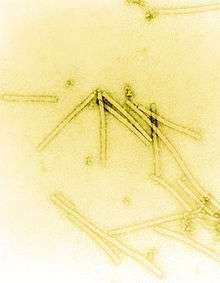 | |
| Electron micrograph of TMV particles stained to enhance visibility at 160,000x magnification | |
| Virus classification | |
| Group: | Group IV ((+)ssRNA) |
| Order: | Unassigned |
| Family: | Virgaviridae |
| Genus: | Tobamovirus |
| Species: | Tobacco mosaic virus |
Tobacco mosaic virus (TMV) is a positive-sense single stranded RNA virus that infects a wide range of plants, especially tobacco and other members of the family Solanaceae. The infection causes characteristic patterns, such as "mosaic"-like mottling and discoloration on the leaves (hence the name). TMV was the first virus ever to be discovered. Although it was known from the late 19th century that an infectious disease was damaging tobacco crops, it was not until 1930 that the infectious agent was determined to be a virus.
History
In 1886, Adolf Mayer first described the tobacco mosaic disease that could be transferred between plants, similar to bacterial infections.[1][2] In 1892, Dmitri Ivanovsky gave the first concrete evidence for the existence of a non-bacterial infectious agent, showing that infected sap remained infectious even after filtering through finest Chamberland filters.[2][3] Later, in 1903, Ivanovsky published a paper describing abnormal crystal intracellular inclusions in the host cells of the affected tobacco plants and argued the connection between these inclusions and the infectious agent.[4] However, Ivanovsky remained rather convinced, despite repeated failures to produce evidence, that the causal agent was an unculturable bacterium, too small to be retained on the employed Chamberland filters and to be detected in the light microscope. In 1898, Martinus Beijerinck independently replicated Ivanovsky's filtration experiments and then showed that the infectious agent was able to reproduce and multiply in the host cells of the tobacco plant.[2][5] Beijerinck coined the term of "virus" to indicate that the causal agent of tobacco mosaic disease was of non-bacterial nature. Tobacco mosaic virus was the first virus to be crystallized. It was achieved by Wendell Meredith Stanley in 1935 who also showed that TMV remains active even after crystallization.[2] For his work, he was awarded 1/3 of the Nobel Prize in Chemistry in 1946,[6] even though it was later shown some of his conclusions (in particular, that the crystals were pure protein, and assembled by autocatalysis) were incorrect.[7] The first electron microscopical images of TMV were made in 1939 by Gustav Kausche, Edgar Pfankuch and Helmut Ruska – the brother of Nobel Prize winner Ernst Ruska.[8] In 1955, Heinz Fraenkel-Conrat and Robley Williams showed that purified TMV RNA and its capsid (coat) protein assemble by themselves to functional viruses, indicating that this is the most stable structure (the one with the lowest free energy). The crystallographer Rosalind Franklin worked for Stanley for about a month at Berkeley, and later designed and built a model of TMV for the 1958 World's Fair at Brussels. In 1958, she speculated that the virus was hollow, not solid, and hypothesized that the RNA of TMV is single-stranded.[9] This conjecture was proven to be correct after her death and is now known to be the + strand.[10] The investigations of tobacco mosaic disease and subsequent discovery of its viral nature were instrumental in the establishment of the general concepts of virology.[2]
Structure
| Tobacco mosaic virus coat protein | |
|---|---|
 A monomeric unit of the tobacco mosaic virus coat protein.[11] | |
| Identifiers | |
| Symbol | CP |
| Entrez | 1494073 |
| UniProt | P03579 |
Tobacco mosaic virus has a rod-like appearance. Its capsid is made from 2130 molecules of coat protein (see image to the left) and one molecule of genomic single strand RNA, 6400 bases long. The coat protein self-assembles into the rod like helical structure (16.3 proteins per helix turn) around the RNA which forms a hairpin loop structure (see the electron micrograph above). The protein monomer consists of 158 amino acids which are assembled into four main alpha-helices, which are joined by a prominent loop proximal to the axis of the virion. Virions are ~300 nm in length and ~18 nm in diameter.[12] Negatively stained electron microphotographs show a distinct inner channel of ~4 nm. The RNA is located at a radius of ~6 nm and is protected from the action of cellular enzymes by the coat protein. There are three RNA nucleotides per protein monomer.[13] X-ray fiber diffraction structure of the intact virus was studied based on an electron density map at 3.6 Å resolution.[11]
Genome
The TMV genome consists of a 6.3-6.5 kb single-stranded (ss) RNA. The 3’-terminus has a tRNA-like structure. The 5’ terminus has a methylated nucleotide cap (m7G5’pppG).[14] The genome encodes 4 open reading frames (ORFs), two of which produce a single protein due to ribosomal readthrough of a leaky UAG stop codon. The 4 genes encode a replicase (with methyltransferase [MT] and RNA helicase [Hel] domains), an RNA-dependent RNA polymerase, a so-called movement protein (MP) and a capsid protein (CP).[15]
Physicochemical properties
TMV is a thermostable virus. On a dried leaf, it can withstand up to 120 degrees Fahrenheit (50 °C) for 30 minutes.
TMV has an index of refraction of about 1.57.[16]
Disease cycle
TMV does not have a distinct overwintering structure. Rather, it will over-winter in infected tobacco stalks and leaves in the soil, on the surface of contaminated seed (TMV can even survive in contaminated tobacco products for many years). With the direct contact with host plants through its vectors (normally insects such as aphids and leaf hoppers), TMV will go through the infection process and then the replication process.
Infection
After its multiplication, it enters the neighboring cells through plasmodesmata. For its smooth entry, TMV produces a 30 kDa movement protein called P30 which enlarge the plasmodesmata. TMV most likely moves from cell-to-cell as a complex of the RNA, P30, and replicase proteins.
It can also spread through phloem for longer distance movement within the plant. Moreover, TMV can be transmitted from one plant to another by direct contact. Although TMV does not have defined transmission vectors, the virus can be easily transmitted from the infected hosts to the healthy plants, by human handling.
Replication
Following entry into its host via mechanical inoculation, TMV uncoats itself to release its viral [+]RNA strand. As uncoating occurs, the MetHel:Pol gene is translated to make the capping enzyme MetHel and the RNA Polymerase. Then the viral genome will further replicate to produce multiple mRNAs via a [-]RNA intermediate primed by the tRNAHIS at the [+]RNA 3' end. The resulting mRNAs encode several proteins, including the coat protein and an RNA-dependent RNA polymerase (RdRp), as well as the movement protein. Thus TMV can replicate its own genome. After the coat protein and RNA genome of TMV have been synthesized, they spontaneously assemble into complete TMV virions in a highly organized process. The protomers come together to form disks or 'lockwashers' composed of two layers of protomers arranged in a helix. The helical capsid grows by the addition of protomers to the end of the rod. As the rod lengthens, the RNA passes through a channel in its center and forms a loop at the growing end. In this way the RNA can easily fit as a spiral into the interior of the helical capsid.[17]
Host and symptoms


Like other plant pathogenic viruses, TMV has a very wide host range and has different effects depending on the host being infected. The tobacco mosaic virus has been known to cause a production loss for flue cured tobacco of up to two percent in North Carolina.[18] It is known to infect members of nine plant families, and at least 125 individual species, including tobacco, tomato, pepper (all members of the useful Solanaceae), cucumbers, and a number of ornamental flowers.[19] There are many different strains. The first symptom of this virus disease is a light green coloration between the veins of young leaves. This is followed quickly by the development of a "mosaic" or mottled pattern of light and dark green areas in the leaves. Rugosity may also be seen where the infected plant leaves display small localized random wrinkles. These symptoms develop quickly and are more pronounced on younger leaves. Its infection does not result in plant death, but if infection occurs early in the season, plants are stunted. Lower leaves are subjected to "mosaic burn" especially during periods of hot and dry weather. In these cases, large dead areas develop in the leaves. This constitutes one of the most destructive phases of tobacco mosaic virus infection. Infected leaves may be crinkled, puckered, or elongated. However, if TMV infects crops like grape and apple, it is almost symptomless.
Environment
TMV is known as one of the most stable viruses. It has a very wide survival range. As long as the surrounding temperature remains below approximately 40 degrees Celsius, TMV can sustain its stable form. All it needs is a host to infect. If necessary, greenhouses and botanical gardens would provide the most favorable condition for TMV to spread out, due to the high population density of possible hosts and the constant temperature throughout the year.
Treatment and management
One of the common control methods for TMV is sanitation, which includes removing infected plants, and washing hands in between each planting. Crop rotation should also be employed to avoid infected soil/seed beds for at least two years. As for any plant disease, looking for resistant strains against TMV may also be advised. Furthermore, the cross protection method can be administered, where the stronger strain of TMV infection is inhibited by infecting the host plant with mild strain of TMV, similar to the effect of a vaccine.
In the past ten years, the application of genetic engineering on a host plant genome has been developed to allow the host plant to produce the TMV coat protein within their cells. It was hypothesized that the TMV genome will be re-coated rapidly upon entering the host cell, thus it prevents the initiation of TMV replication. Later it was found that the mechanism that protects the host from viral genome insertion is through gene silencing.[20]
Scientific and environmental impact

The large amount of literature about TMV and its choice for many pioneering investigations in structural biology (including X-ray diffraction), virus assembly and disassembly, and so on, are fundamentally due to the large quantities that can be obtained, plus the fact that it does not infect animals. After growing a few infected tobacco plants in a greenhouse and a few simple laboratory procedures, a scientist can easily produce several grams of the virus. As a result of this, TMV can be treated almost as an organic chemical, rather than an infective agent.
Investigational uses
Due to its cylindrical high aspect ratio, self-assembling nature, and ability to incorporate metal coatings (nickel and cobalt) into its shell, TMV is an ideal candidate to be incorporated into battery electrodes[21] Addition of TMV to a battery electrode increases the reactive surface area by an order of magnitude, resulting in an increase in the battery's capacity by up to six times compared to a planar electrode geometry.[21][22]
References
- ↑ Mayer, Adolf (1886). "Über die Mosaikkrankheit des Tabaks.". Die Landwirtschaftliche Versuchs-stationen (in German) 32: 451–467. Translated into English in Johnson, J., Ed. (1942) Phytopathological classics (St. Paul, Minnesota: American Phytopathological Society) No. 7, pp. 11–24.
- 1 2 3 4 5 Zaitlin, Milton (1998). "The Discovery of the Causal Agent of the Tobacco Mosaic Disease" (PDF). In Kung, S. D.; Yang, S. F. Discoveries in Plant Biology. Hong Kong: World Publishing Co. pp. 105–110. ISBN 978-981-02-1313-8.
- ↑ Iwanowski, D. (1892). "Über die Mosaikkrankheit der Tabakspflanze". Bulletin Scientifique publié par l'Académie Impériale des Sciences de Saint-Pétersbourg / Nouvelle Serie III (in German and Russian) (St. Petersburg) 35: 67–70. Translated into English in Johnson, J., Ed. (1942) Phytopathological classics (St. Paul, Minnesota: American Phytopathological Society) No. 7, pp. 27–30.
- ↑ Iwanowski, D. (1903). "Über die Mosaikkrankheit der Tabakspflanze". Zeitschrift für Pflanzenkranheiten und Pflanzenschutz (in German) 13: 1–41.
- ↑ Beijerinck, M. W. (1898). "Über ein Contagium vivum fluidum als Ursache der Fleckenkrankheit der Tabaksblätter". Verhandelingen der Koninklyke akademie van Wettenschappen te Amsterdam (in German) 65: 1–22. Translated into English in Johnson, J., Ed. (1942) Phytopathological classics. (St. Paul, Minnesota: American Phytopathological Society) No. 7, pp. 33–52 (St. Paul, Minnesota)
- ↑ Wendell M. Stanley – Biography
- ↑ Kay LE (September 1986). "W. M. Stanley's crystallization of the tobacco mosaic virus, 1930–1940". Isis 77 (288): 450–72. doi:10.1086/354205. JSTOR 231608. PMID 3533840.
- ↑ Kausche GA, Pfankuch E, Ruska H (May 1939). "Die Sichtbarmachung von pflanzlichem Virus im Übermikroskop". Naturwissenschaften 27 (18): 292–9. doi:10.1007/BF01493353.
- ↑ Maddox, Brenda (2002). Rosalind Franklin, the Dark Lady of DNA. Harper Collins. ISBN 0-06-018407-8.
- ↑ http://www.agls.uidaho.edu/ebi/vdie/descr803.htm#Properties
- 1 2 PDB: 1VTM; Namba K, Stubbs G (March 1986). "Structure of tobacco mosaic virus at 3.6 A resolution: implications for assembly". Science 231 (4744): 1401–6. doi:10.1126/science.3952490. PMID 3952490.
- ↑ Stryer, Lubert (1988). Biochemistry. San Francisco: W.H. Freeman. ISBN 0-7167-1843-X.
- ↑ Klug A (March 1999). "The tobacco mosaic virus particle: structure and assembly". Philosophical Transactions of the Royal Society B 354 (1383): 531–5. doi:10.1098/rstb.1999.0404. PMC 1692534. PMID 10212932.
- ↑ Expasy Viralzone Tobamovirus
- ↑ Gergerich, R.C., and V. V. Dolja. 2006. Introduction to Plant Viruses, the Invisible Foe. The Plant Health Instructor. DOI: 10.1094/PHI-I-2006-0414-01
- ↑ Ashkin, Arthur; Dziedzic, JM (March 20, 1987). "Optical Trapping and Manipulation of Viruses and Bacteria". Science 235 (4795): 1517–1520. doi:10.1126/science.3547653. PMID 3547653.
- ↑ Chris Woolverton; Joanne Willey; Sherwood, Linda (2008). Prescott's Microbiology (7th ed.). Boston: McGraw Hill Higher Education. pp. 464–5. ISBN 0-07-110231-0.
- ↑ "Control of Tobacco Mosaic Virus on Flue-Cured Tobacco". Retrieved 2009-02-21.
- ↑ Tomato-Tobacco Mosaic Virus Disease
- ↑ Agrios, George (2005). Plant Pathology, 5th Edition. Burlington, MA: Elsevier Academic Press. p. 320. ISBN 0-12-044565-4.
- 1 2 Gerasopoulos, K.; McCarthy, M.; Royston, E.; Culver, J.N.; Ghodssi, R. (January 13–17, 2008). "Microbatteries with Tobacco Mosaic Virus Templated Electrodes". Micro Electro Mechanical Systems, 2008 (Tucson, USA): 960–963. doi:10.1109/MEMSYS.2008.4443817. ISBN 978-1-4244-1792-6. ISSN 1084-6999. Retrieved December 10, 2010.
- ↑ Atanasova, P.; Rothenstein, D.; Schneider, J. J.; Hoffmann, R. C.; Dilfer, S.; Eiben, S.; Wege, C.; Jeske, H.; Bill, J.; et al. (2011). "Virus-templated synthesis of ZnO nanostructures and formation of field-effect transistors". Adv. Mat. 23: 4918–4922. doi:10.1002/adma.201102900.
Further reading
- Creager, Angela N. H. (2002). The life of a virus: tobacco mosaic virus as an experimental model, 1930–1965. Chicago: University of Chicago Press. ISBN 0-226-12026-0.
- Flue-cured tobacco field manual published R.J.Reynolds Tobacco Company, Winston-Salem, North Carolina, 1995
External links
| Wikimedia Commons has media related to Tobacco mosaic virus. |
- Description of plant viruses – TMV – contains information on symptoms, hosts species, purification etc.
- Further information
- Electron microscope image of TM
