Tishchenko reaction
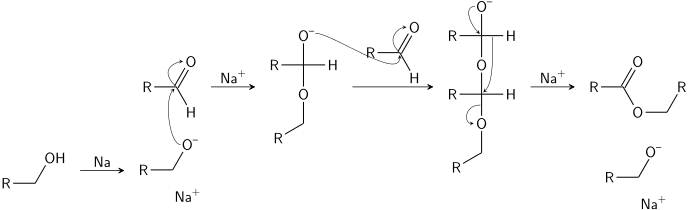
The Tishchenko reaction is an organic chemical reaction that involves disproportionation of an aldehyde lacking a hydrogen atom in the alpha position in the presence of an alkoxide.[1] The reaction product is an ester. Catalysts are aluminium alkoxides or sodium alkoxides.
In the related Cannizzaro reaction the base is sodium hydroxide and then the oxidation product is a carboxylic acid and the reduction product is an alcohol.
Examples
- Benzaldehyde reacts with sodium benzyloxide (generated from sodium and benzyl alcohol) to generate benzyl benzoate.[2]

- The Tishchenko reaction of acetaldehyde gives the commercially important solvent ethyl acetate; it is catalyzed by aluminium alcoholate.[3]
- The Tishchenko reaction is used to obtain isobutyl isobutyrate, a specialty solvent.[4]
- Hydroxypivalic acid neopentyl glycol ester is produced by a Tishchenko reaction from hydroxypivaldehyde in the presence of a basic catalyst (e.g., aluminium oxide).[5]
- The Tishchenko reaction of paraformaldehyde in the presence of aluminum methylate or magnesium methylate forms methyl formate.[6]
- Paraformaldehyde reacts with boric acid to form methyl formate.[7] The key step in the reaction mechanism for this reaction is a 1,3-hydride shift in the hemiacetal intermediate formed from two successive nucleophilic addition reactions, the first one from the catalyst. The hydride shift regenerates the alkoxide catalyst.
Related reactions
- Aldol–Tishchenko reaction
- Baylis–Hillman reaction
- Cannizzaro reaction
- Meerwein–Ponndorf–Verley reduction
- Oppenauer oxidation
References
- ↑ V. Tishchenko (1908), J. Russ. Phys. Chem. Soc 38: 355, 482 Missing or empty
|title=(help) - ↑ Kamm, O.; Kamm, W. F. (1941). "Benzyl benzoate". Org. Synth.; Coll. Vol. 1, p. 104
- ↑ Marc Eckert, Gerald Fleischmann, Reinhard Jira, Hermann M. Bolt, Klaus Golka (2007), "Acetaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 4
- ↑ Boy Cornils, Richard W. Fischer, Christian Kohlpaintner (2007), "Butanals", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 4
- ↑ Peter Werle, Marcus Morawietz (2007), "Alcohols, Polyhydric", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 6
- ↑ Günther Reuss, Walter Disteldorf, Armin Otto Gamer, Albrecht Hilt (2007), "Formaldehyde", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 5
- ↑ Paul R. Stapp (1973). "Boric acid catalyzed Tishchenko reactions". J. Org. Chem. 38 (7): 1433–1434. doi:10.1021/jo00947a049.
This article is issued from Wikipedia - version of the Tuesday, December 08, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.