Tirapazamine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
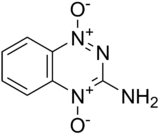
4-hydroxy-1-oxido-1,2,4-benzotriazin-1-ium-3-imine | |
| Identifiers | |
| CAS Number |
27314-97-2 |
| ATC code | None |
| PubChem | CID 33776 |
| ChemSpider |
10437748 |
| UNII |
1UD32YR59G |
| KEGG |
D06167 |
| ChEBI |
CHEBI:78887 |
| ChEMBL |
CHEMBL50882 |
| Chemical data | |
| Formula | C7H6N4O2 |
| Molar mass | 178.148 g/mol |
| |
| |
| | |
Tirapazamine (SR-4233) is an experimental anticancer drug that is activated to a toxic radical only at very low levels of oxygen (hypoxia). Such levels are common in human solid tumors, a phenomenon known as tumor hypoxia. Thus, tirapazamine is activated to its toxic form preferentially in the hypoxic areas of solid tumors. Cells in these regions are resistant to killing by radiotherapy and most anticancer drugs. Thus the combination of tirapazamine with conventional anticancer treatments is particularly effective. As of 2006, tirapazamine is undergoing phase III testing in patients with head and neck cancer and gynecological cancer, and similar trials are being undertaken for other solid tumor types.[1][2]
Chemically it is an aromatic heterocycle di-N-oxide. Its full chemical name is 3-amino-1,2,4-benzotriazine-1,4 dioxide. Originally it was prepared in a programme screening for new herbicides in 1972. Its clinical use was first described by Zeman et al. in 1986.[3] While tirapazamine has had only limited effectiveness in clinical trials,[4] it has been used as a lead compound to develop a number of newer compounds with improved anti-cancer properties.[5]
An update of a Phase III trial (Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group) found no evidence that the addition of TPZ to chemoradiotherapy, in patients with advanced head and neck cancer not selected for the presence of hypoxia, improved overall survival.[6]
Two possible molecular mechanisms of TPZ, for generating reactive oxygen species which causes DNA strand break, have been considered widely. In hypoxia, under bioreductive condition, it has been observed that TPZ primarily produces hydroxyl or and benzotriazinyl radicals as the DNA damaging reactive species.[7][8]
A new clinical phase I trial of Tirapazamine combined with embolization in liver cancer has been received in June, 2014. This study will help to optimize the safe tollerable dose of TPZ, when it is administered with embolization in liver cancer. [9] Treatment of solid tumors is compliated by the fact that these are often poorly provided with blood vessels, thus limiting their exposure to cytotoxic agents. Attempts have, however, been made to take advantage of the resulting hypoxic environment by designing drugs that are nonreactive until they are reduced to reactive species in oxygen-deficient tissues. This, it is hoped, will lead to enhanced selectivity. The azaquinoxaline dioxide function on the antineoplastic agent tirapazamine, for example, has been shown to give reactive nitroxide radicals on reduction.
Synthesis
The first step in the synthesis, condensation of 2-nitroaniline (1) with cyanamide, probably involves initial formation of a guanidine such as 2. This then cyclizes to the heterocycle 3. Oxidation with hydrogen peroxide then completes the preparation of tirapazamine (4).
References
- ↑ Denny, WA (2004). "Prospects for hypoxia-activated anticancer drugs". Current Medicinal Chemistry 4 (5): 395–9. doi:10.2174/1568011043352812. PMID 15379691.
- ↑ Gandara, DR; Lara Jr, PN; Goldberg, Z; Le, QT; Mack, PC; Lau, DH; Gumerlock, PH (2002). "Tirapazamine: prototype for a novel class of therapeutic agents targeting tumor hypoxia". Seminars in oncology 29 (1 Suppl 4): 102–9. doi:10.1053/sonc.2002.31531. PMID 11894020.
- ↑ Zeman, EM; Brown, JM; Lemmon, MJ; Hirst, VK; Lee, WW (1986). "SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells". International journal of radiation oncology, biology, physics 12 (7): 1239–42. doi:10.1016/0360-3016(86)90267-1. PMID 3744945.
- ↑ Reddy, SB; Williamson, SK (January 2009). "Tirapazamine: a novel agent targeting hypoxic tumor cells". Expert Opinion on Investigational Drugs 18 (1): 77–87. doi:10.1517/13543780802567250. PMID 19053884.
- ↑ Hay, MP; Hicks, KO; Pchalek, K; Lee, HH; Blaser, A; Pruijn, FB; Anderson, RF; Shinde, SS; et al. (November 2008). "Tricyclic 1,2,4Triazine 1,4-Dioxides As Hypoxia Selective Cytotoxins". Journal of Medicinal Chemistry 51 (21): 6853–65. doi:10.1021/jm800967h. PMC 2690574. PMID 18847185.
- ↑ Rischin, D; Peters, LJ; O'Sullivan, B; Giralt, J; Fisher, R; Yuen, K; Trotti, A; Bernier, J; et al. (2010). "Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group". Journal of clinical oncology 28 (18): 2989–95. doi:10.1200/JCO.2009.27.4449. PMID 20479425.
- ↑ Junnotula, V; Sarkar, Ujjal; S, Sinha; Kent, Gates (2009). "Initiation of DNA strand cleavage by 1,2,4-benzotriazine 1,4-dioxide antitumor agents: mechanistic insight from studies of 3-methyl-1,2,4-benzotriazine 1,4-dioxide". J Am Chem Soc 131 (13): 1015–24. doi:10.1021/ja8049645. PMID 19117394..
- ↑ Brown, Martin; Wilson, William (2004). "Exploiting tumour hypoxia in cancer treatment". Nature Reviews Cancer 4 (6): 437–447. doi:10.1038/nrc1367. PMID 15170446.
- ↑ "Dose-defining Study of Tirapazamine Combined With Embolization in Liver Cancer". clinicaltrials.gov.
- ↑ Mason, J. C.; Tennant, G. (1970). "Heterocyclic N-oxides. Part VI. Synthesis and nuclear magnetic resonance spectra of 3-aminobenzo-1,2,4-triazines and their mono- and di-N-oxides". Journal of the Chemical Society B: Physical Organic: 911. doi:10.1039/J29700000911.
- ↑ Robbins, R. F.; Schofield, K. (1957). "623. Polyazabicyclic compounds. Part II. Further derivatives of benzo-1 : 2 : 4-triazine". Journal of the Chemical Society (Resumed): 3186. doi:10.1039/JR9570003186.
- ↑ K. Ley et al., DE 2204574; eidem, U.S. Patent 3,868,371 (1973, 1975 both to Bayer).
External links
- Clinical trial number NCT00033410 for "Chemotherapy, Tirapazamine, and Radiation Therapy in Treating Patients With Non-Small Cell Lung Cancer" at ClinicalTrials.gov
