Ticks of domestic animals
Ticks of domestic animals directly cause poor health and loss of production to their hosts by many parasitic mechanisms. Ticks also transmit numerous kinds of viruses, bacteria, and protozoa between domestic animals. These microbes cause diseases which can be severely debilitating or fatal to domestic animals, and may also affect humans. Ticks are especially important to domestic animals in tropical and subtropical countries, where the warm climate enables many species of ticks to flourish. Also, the large populations of wild animals in warm countries provide a reservoir of ticks and infective microbes that spread to domestic animals. Farmers of livestock animals use many methods to control ticks, and related treatments are used to reduce infestation of companion animals. Veterinarians and animal health agencies work at private, national, and international scales to reduce the harm caused by ticks and their associated diseases.

Range of ticks affecting domestic animals
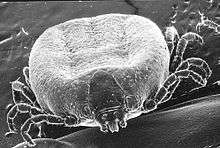
Ticks are invertebrate animals in the phylum Arthropoda, and are related to spiders. Ticks are in the subclass Acari which consists of many orders of mites and one tick order, the Ixodida. Some mites are parasitic, but all ticks are parasitic feeders on blood. Some species of mites may be mistaken for larval ticks at infestations, but their feeding mechanisms are distinctive. All ticks have an incomplete metamorphosis: after hatching from the egg, a series of similar stages (= instars) develops from a six-legged larva, to eight-legged nymph, and then a sexually developed, eight-legged adult. Between each stage is a molt (ecdysis) which enables the developing tick to expand within a new external skeleton. Ticks are grouped in three families of which two have genera of importance to domestic animals, as follows.[1] The family Argasidae contains the important genera Argas, Ornithodoros, and Otobius. These genera are known as soft ticks because their outer body surfaces are like hard plates. The family Ixodidae contains the important genera Amblyomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Margaropus, and Rhipicephalus. Also, the important boophilid ticks, formerly of the genus Boophilus, are now classified as a subgenus within the genus Rhipicephalus. These genera are known as hard ticks because their outer surfaces have hard plates. Within these 10 genera are, very approximately, 100 species of importance to domestic animals.[2] Some of these species are also important to humans. The only countries that do not have some kind of problem with ticks on domestic animals are those that are permanently cold.
Typical ticks of domestic animals
Amblyomma and Rhipicephalus ixodid ticks

Amblyomma species are widespread on domestic animals throughout tropical and subtropical regions. Typical Amblyomma species are: Amblyomma americanum, the lone star tick of southern and eastern USA; Am. cajennense, the Cayenne tick of South America and southern USA; Am. variegatum, the bont tick of Africa and the Caribbean. A typical Rhipicephalus species is Rhipicephalus sanguineus, the tropical dog tick, specialized to feed only on dogs. It is distributed globally throughout the warm countries, wherever humans with their dogs live. Typical Rhipicephalus species that feed on cattle in Africa are R. appendiculatus, the brown ear-tick, and R. evertsi evertsi, the red-legged tick. Rhipicephalus (Boophilus) microplus is the most important tick of cattle in many tropical and subtropical countries to where it spread from Southeast Asia on transported cattle.
Three-host lifecycle of Amblyomma and many Rhipicephalus species
Following one individual tick: the eggs laid on the ground hatch and the larvae wait for or actively seek a host (questing behaviour). [1] The larva feeds, detaches from its host, molts into a nymph when on the ground and quests by crawling on the ground or waiting on vegetation. The nymph feeds and repeats the same process as the larva, but will emerge having developed the anatomy of either an adult female or male. Adults quest similarly to nymphs. Most hard ticks have a three-host lifecycle. The female attaches only to a species of host that it is adapted to for reproduction. The female engorges on much blood, expanding greatly, then detaches and converts the blood meal into eggs which are laid on the ground. Females of large species of Amblyomma engorge to a weight of 5 g and lay 20,000 eggs. The female dies after this single egg-laying. The male takes repeated small meals of blood and attempts to mate repeatedly whilst on the same host. Feeding times range over: larvae 4–7 days, nymphs 5–10 days, and adults 8 to 20 days. The time spent molting and questing off the host can occupy the remainder of 6 to 18 months for a single tick to complete its lifecycle. The lifecycle timing is often expanded by diapause (ceased development or activity) in adaptation to seasonal variation of moisture and heat. Ticks are highly adapted for long-term survival off the host without feeding and can extract moisture directly from humid air. However, survival is greatly reduced by excess heat, dryness, and lack of suitable hosts to which to attach. Survival on the host is also greatly reduced by grooming and by hypersensitive immune reactions in the skin against the feeding of the ticks. [1]
Boophilid ticks, a subgenus within Rhipicephalus

These ticks, commonly known as cattle ticks or blue ticks, have a highly characteristic morphology and one-host lifecycle. They are economically important to cattle rearing industry by causing direct parasitic losses and by transmission of microbes. In addition to Rhipicephalus microplus, species of most importance to domestic animals are R. annulatus, which is widespread in tropical and subtropical countries, and R. decoloratus which occurs in Africa.
One-host lifecycle of R. microplus
These ticks are adapted to the advantages of specialising to feed on cattle and with all the feeding stages occurring on one individual host in a rapid sequence. [1] They also can survive by feeding on deer or some wild bovid hosts. Infestation starts when larvae on vegetation attach to a new host. When a larva feeds, it molts at the site where it feeds and emerges as a nymph. The nymph feeds at the same site or close by and molts where it feeds. It emerges from molt as either an adult female or male. The female’s single large blood meal is converted into a batch of 2000 eggs. The males take several small meals of blood to support their repeated attempts at mating. The molts are rapid and the next stage remains in the hair coat to start feeding again. The combined feeding and molting periods take about 21 days. The engorged female drops from the host, hides under litter on soil surface, lays one batch of eggs, and then dies. When eggs hatch, the larvae crawl up grass stems and wait until they can attach to passing cattle.
Hyalomma ticks

This genus contains many species of hard ticks important to domestic animals in hot dry regions in Africa, the Mediterranean basin, the Middle East, Pakistan, India,[3] and through to China. Typical species are Hyalomma anatolicum anatolicum, Hy. marginatum rufipes, Hy. truncatum, and Hy. detritum detritum, which feed as adults on cattle, sheep, and goats. Hy. dromedarii is specialized to feed on dromedary camels. Hyalomma ticks are adapted to live in regions with large seasonal variation of temperature and low rainfall. Diapause is an important mechanism to adjust to these climates. Another adaptation is to have a lifecycle within one species that can be two-host or three-host. For example: Hy. a. anatolicum may feed on a hare, molt on the hare, and feed again on the same individual hare, detach and molt to an adult and then feed on a cow; this is a two-host lifecycle, or it may feed as a larva on a gerbil, then as a nymph on a cow, and then as an adult on another cow in a three-host lifecycle. Furthermore, this tick commonly feeds as a three-host tick with larvae, nymphs, and adults feeding on separate individual dairy cows confined to cattle housing in zero-grazing systems.
Argas and Ornithodoros soft ticks

Argas persicus, the fowl tick, is a major pest of poultry birds. The tampan ticks within Ornithodoros moubata complex of species infest domestic pigs and also feed on humans. Ornithodoros savignyi is often found in large numbers at enclosures where camels and cattle are herded.
Multi-host lifecycle of O. moubata

Argasidae soft ticks have different lifecycles from Ixodidae hard ticks, and these are very variable between species. [1] Typically, in Ornithodoros, a larva hatches from an egg laid in the nest or resting place of the host. The larva does not feed, but directly molts into the first nymph stage. This stage feeds then molts into the next nymph stage. Feeding by soft ticks is generally completed within minutes rather than days as with hard ticks. Depending on circumstances, four or five nymph stages occur, each progressively larger. Finally, a molt produces an adult female or male. The female takes repeated blood meals that are small compared to a female hard tick. Each blood meal is converted to a small batch of eggs. The male feeds sufficiently to support its mating. The lifecycle of Argas persicus is similar, but the larva feeds on blood of its bird host, remaining attached around 7 days.
Other groups of ticks
Other genera with species that are often of high local importance to domestic animals include these examples: Ixodes (Ixodes ricinus, the deer tick of Europe; Ixodes scapularis, the black-legged tick of North America; Ixodes holocyclus, the paralysis tick of Australia). Haemaphysalis (Ha. leachii, the yellow dog tick of the tropics). Dermacentor (Dermacentor andersoni, the Rocky Mountain wood tick; Dermacentor variabilis, the American dog tick; D. reticulatus, the ornate dog tick of Europe). Dermacentor nitens the tropical horse tick of the Americas has a one-host lifecycle similar to the boophilids. Margaropus winthemi, the beady-legged tick, infests horses and cattle in South Africa. The soft tick Otobius megnini, the spinose ear tick, has its nymphs feeding within the ear canal of many species of domestic animals. Adults of Ot. megnini do not feed. This tick occurs in the Americas and has spread to Africa and Asia.
Types of harm directly caused to domestic animals by feeding of ticks
Biting stress and lost production
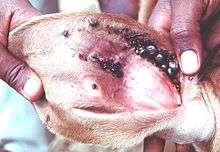
When a hard tick pierces the skin of its host, initially little or no pain is caused. Later, during the prolonged feeding of ticks, inflammation is caused at the wound, followed by acquired immune reactions in the skin (dermal hypersensitivities types 1 and 4) to the foreign proteins in tick saliva. This defense by the host is generally effective, but at the cost of pruritus (itch) and pain at the feeding site. Infestations of ticks on domestic and wild animals can build up to very high levels. This occurs on a minor proportion of individuals in the herd whilst most individual animals have low infestations (an aggregated or overdispersed distribution). On a herd basis, the accumulated effect of this biting stress can cause loss of appetite (anorexia) and loss of blood. These two losses result in reduced feed intake and anemia; combined, they cause a lower rate of growth or of milk production compared to hosts without tick infestation.[4][5] The feeding of soft ticks can cause severe biting stress because of the pain whilst they feed; Ornithodoros savignyi is one notorious example.
Physical damage

At each feeding site of hard ticks, granuloma and wound healing produce a scar that remains for years after the tick has detached. When the skin of livestock animals is made into leather, these scars remain as blemishes that reduce the value of the leather. Larger ticks cause obstructive and painful damage, such as Amblyomma variegatum adults which often feed on udders of cattle and reduce suckling by the calves. Hyalomma truncatum adults feed on the feet of sheep and goats, causing lameness. Wounds caused by dense clusters of adult ticks can make the host susceptible to infestation with larvae of flesh-eating myiasis flies, such as the screw-worm, Cochliomyia hominivorax. [5] [6][7]
Poisoning

When ticks feed, they secrete saliva containing powerful enzymes and substances with strong pharmacological properties to maintain flow of blood and reduce host immunity. Sometimes, this causes a poisoning of the host. This is not because of a functional toxin in the sense that snake poison is functional for the snake. However, the result can be various forms of toxaemia caused by a variety of ticks. A moist eczema, sometimes with hair loss (alopecia) known as sweating sickness in cattle is caused by Hyalomma truncatum. Tick paralysis can be life-threatening and is caused in sheep by feeding of Ixodes rubicundus of South Africa; in cattle caused by Dermacentor andersoni in North America; in cattle, dogs and humans caused by the Australian paralysis tick, Ixodes holocyclus. [1] [8][9][10]
Types of harm caused by microbes transmitted by ticks
These are numerous; typical examples follow. Because ticks feed repeatedly and only on blood, and have long lives, they are suitable hosts for many types of microbes. The microbes exploit the ticks for transmission between one domestic animal and another. Ticks are thus known as vectors (transmitters) of microbes. If the microbes cause pathological changes, they are known as pathogens. Most of these parasitic relationships are highly developed with a strict biological relationship between the microbe and the tick’s gut and salivary glands. However, some microbes, such as Anaplasma marginale and A. centrale, can also be transmitted by biting flies, or by blood on injection needles (iatrogenic transmission). A characteristic of diseases caused by tick-transmitted microbes is the epidemiological state of endemic stability that commonly develops in herds or flocks of livestock. This stability is due to high levels of immunity to the microbes developing as a result of survival through early infection from infected ticks. The ticks are often constantly present and long-lived. Acquisition of immunity may be aided by the protection of antibodies in the mother’s colostrum (first milk). Some microbes causing diseases associated with ticks are not transmitted by the ticks. For example, the skin disease dermatophilosis of cattle, sheep, and goats is caused by the bacterium Dermatophilus congolensis, which is transmitted by simple contagion. But when Amblyomma variegatum adult ticks are also feeding and causing a systemic suppression of immunity in the host, then dermatophilosis becomes severe or even fatal.[11]
Viral diseases
The virus of Nairobi sheep disease in East Africa is transmitted by Rhipicephalus ticks. African swine fever is naturally transmitted between wild species of the pig family by feeding of Ornithodoros moubata group ticks. This pattern of transmission can expand to include domestic pigs. However, within groups of domestic pigs, the virus can also be transmitted by contagion. Crimean-Congo hemorrhagic fever virus is transmitted between many mammal species by Hyalomma truncatum, Hy. m. rufipes, and Hy. m. turanicum over a wide area of Africa, Europe, and Asia. In cattle and sheep, it causes mild fever and its main importance is when it spreads to humans (zoonosis) by feeding of the larvae or nymphs of these ticks. [1] [12]
Bacterial diseases

Borrelia bacteria are well described elsewhere in association with Ixodes ticks for causing Lyme disease in humans, but this disease also affects domestic dogs. Borrelia anserina is transmitted by Argas persicus to poultry, causing avian borreliosis in a wide spread of tropical and subtropical countries.[13] Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) is a bacterium of deer that spreads to sheep where it causes tick-borne fever in Europe, resulting in abortion by ewes and temporary sterility of rams. This bacterium invades and proliferates in neutrophil cells of the blood. This depletes these antibacterial cells and renders the host susceptible to opportunistic infections by Staphylococcus aureus bacteria which invade joints and cause the crippling disease of sheep called tick pyaemia. Anaplasma marginale infects marginal areas of red blood cells of cattle and causes anaplasmosis wherever boophilid ticks occur as transmitters. Anaplasma centrale tends to infect the central region of red blood cells, and is sufficiently closely related to An. marginale to have been used from long ago as a live vaccine to protect cattle against the more virulent An. marginale. Sheep and goats suffer disease from infection with Anaplasma ovis which is transmitted similarly to the anaplasmas described above. Ehrlichia ruminantium (formerly Cowdria ruminantium) is transmitted mainly by Amblyomma hebraeum and Am. variegatum in Africa, causing the severe disease heartwater in cattle, sheep, and goats. This disease is named after the prominent sign of pericardial edema. The bacteria infect the brain, causing prostration. Heartwater also occurs on the Caribbean islands, having spread there on shipments of cattle from Africa about 150 years ago, before anything was known of tick transmitted microbes. [11] [14]
Protozoal diseases

Babesia bovis protozoa are transmitted by R. microplus and cause babesiosis or redwater fever in cattle throughout the tropics and subtropics wherever this boophilid species occurs. The less pathogenic Ba. bigemina is transmitted by R. microplus and R. decoloratorus. Development of Babesia in the tick is complex and includes sexual reproduction. These Babesia are transmitted from adult female boophilid ticks to the next generation, as larvae, by infection of the eggs. This is known as transovarian transmission; it provides the only opportunity for transmission through one-host ticks. Other species of Babesia are transmitted by three-host ticks in ways similar to Theileria protozoa, as described below. In cattle, infection of the red blood cells may grow rapidly to create a potentially fatal inflammatory crisis of the blood. The name redwater (coloured urine) derives from the hemoglobinuria caused by the destruction of red blood cells infected with the merozoite stage of Babesia; anemia results from the same destruction. Horses suffer babesiosis or biliary fever when infected by Ba. equi or B. caballi. This occurs in many countries where vector ticks are found, such as R. e. evertsi, Hy. truncatum, and D. nitens. Dogs are at risk from severe infection with Ba. canis and its subspecies, transmitted by the dog ticks R. sanguineus, D. reticulatus, and Ha. leachi. Domestic cats become infected with Ba. felis and Ba. cati from feeding ticks. Cytauxzoon felis is a protozoan related to Babesia and Theileria. It is transmitted by the American dog tick, Dermacentor variabilis. This microbe circulates between wild bobcats in southern USA, causing little apparent disease. If it infects domestic cats, it causes a cytauxzoonosis that is eventually fatal. [11]

Theileria annulata is a protozoan closely related to Babesia. In cattle, it causes the disease tropical theileriosis throughout a long arc of countries from Morocco across to China.[15] Theileria parva is the causative microbe of East Coast fever of cattle in Eastern, Central, and Southern Africa. Theileria species infect monocytic white blood cells of their hosts. The infected cells are induced to divide by the Theileria, which then proliferates within each daughter cell, in a rapidly expanding infection. This causes multiple inflammatory crises, of which pulmonary edema is a predominant cause of death. Theileria annulata is transmitted by Hy. a. anatolicum, Hy. detritum, and other hyalommas. Theileria parva is transmitted predominantly by Rhipicephalus appendiculatus. Because this is a three-host feeding tick, the opportunities for transmission are from infected cow to feeding larva then through the molt into the nymph which feeds and transmits. Similarly, also, transmission can be from feeding nymphs to infected adult. This is known as transstadial transmission, and no transovarian transmission occurs in this case. The development of Theileria in ticks includes sexual reproduction which enables generation of new variants that can evade the immune mechanisms of cattle.[16]
Disease control methods
Treatment with chemical pesticide against ticks

Synthetic chemical pesticides specific for ticks (acaricides) are suspended in water for application to the hair coat of domestic animals. Cattle can be immersed in dip-baths containing dipwash, or soaked using a pressurized spray-race made of metal tubing and nozzles. Sheep can be treated in smaller dips or showers. Acaricides can be applied to dogs in watery shampoo formulations. Acaricide-active ingredients are usually soluble in oil. This makes them suitable for concentrated oily formulations which spread from a pour-on applicator over the hair coat. Alternatively, some acaricides are incorporated in polyvinylchoride plastic ear tags for cattle, or collars for dogs. [7] [17][18] Modern acaricides belong to the general classes of organophosphates (example chlorfenvinphos), formamidines (example amitraz), synthetic pyrethroids (example flumenthrin), phenylpyrazoles (example fipronil), and benzylphenyl ureas (example fluazuron). [2] When correctly applied, they can be highly effective. Problems with acaricides are: danger of acute poisoning of treated animals and human staff; residues contaminating meat and milk; environmental contamination especially water sources; resistance that ticks acquire to acaricides; and cost of application. Cost and contamination can be reduced by seasonal timing of application (strategic treatment) based on ecological knowledge. Prediction of best times for treatment can be made using computerized models of the population dynamics of ticks.[19][20][21] Farmers lacking sufficient cash to purchase manufactured synthetic acaricides often use various herbal treatments, locally available. Nicotine as treated tobacco leaf is an example, but such unregistered preparations require careful use to avoid poisoning or skin damage.
Biological methods against ticks
Breeding for resistant cattle has been successful for their ability to acquire strong immune resistance to Rhipicephalus microplus following natural exposure to these ticks.[22] Commercial breeds of cattle (examples: Australian Friesian Sahiwal and Australian Milking Zebu) are successful in the relevant environment. Only a few commercial breeds of tick-resistant cattle are available. For farms infested with R. microplus in Australia and South America, rotation of pasture can kill the questing larvae by starvation and desiccation.[23] However, farmers find the priority to provide their stock with good feed often conflicts with such controls.[24] Ticks affecting dogs and other companion animals around private houses can be reduced by clearing of vegetation and leaf litter, mowing grass short, and fencing out deer and other wild animals that bring in ticks.
Drug treatment against microbes
Antibiotics with efficacy against bacterial pathogens transmitted by ticks include: tetracycline, penicillin, and doxicycline. Against Babesia protozoa are imidocarb and diminazine, both of which can be used to treat patent clinical infections. Against Theileria are parvaquone and halofuginone, both effective for clinical cases. These drugs are usually administered to treat diagnosed cases, but the timing of treatment then becomes critical. Problems with drug treatment include the development of resistance by the microbes and cost. Also, treatment does not necessarily fully clear infections and this may lead to persistent subclinical infections which remain infective to more ticks (carrier infections); this may be considered unsafe in some situations. [2] [9]
Vaccination against microbes and ticks
Vaccination against An. marginale can be done using live strains of the cross-reactive An. centrale. Vaccines are available on a commercial basis to immunize cattle against Babesia bovis. This is made by serial infection of calves to attenuate the virulence of the strain of Babesia, followed by splenectomy to produce many of the piroplasm stage in blood, which is then bottled for use. The vaccine is delivered containing the live protozoa to induce immunity without acute disease.[25] Theileria annulata can be grown and attenuated in virulence by means of infecting cell cultures with the schizont stage of the protozoan. This is delivered as a frozen vaccine from which live parasites are thawed out before injection.[26] Vaccines are often highly effective, but the live parasite vaccines have problems of potential contamination with other microbes and induction of a carrier state which may be unwanted. Intensive attempts are being made to develop vaccines to control these diseases using recombinant DNA techniques to synthesize the relevant antigens. However, as with vaccines against human malaria, they are currently proving difficult.[27]
A commercial vaccine was developed in Australia against Rh. microplus.[28] It acts against a glycoprotein molecule that is exposed on the outer membrane of digestive cells of the gut of feeding ticks. This molecule is synthesized using recombinant DNA technique to make the antigen of the vaccine. Vaccinated cattle develop antibodies circulating in their blood. When the Rh. microplus female ticks engorge with blood, the antibody reacts with the natural antigen in their guts so strongly that digestion is disrupted and the reproductive rate of the ticks is reduced. This vaccine is manufactured in Australia and a closely similar vaccine is manufactured in Cuba.
References
- ↑ Sonenshine, D.E. (2014). Biology of ticks (vols 1&2). New York: Oxford University Press. ISBN 978-0-19-974405-3.
- ↑ Taylor, M.A. (2007). Veterinary parasitology. Oxford: Blackwell Publishing. ISBN 978-1-4051-1964-1.
- ↑ Geeverghese, G. (1987). The Indian Hyalomma ticks. New Delhi: Indian Council of Agricultural Research.
- ↑ Jonsson, N.N. (2006). "The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses". Veterinary Parasitology 137 (1–2): 1–10. doi:10.1016/j.vetpar.2006.01.010. PMID 16472920.
- ↑ Pegram, R.G. (1991). "Studies on the economics of ticks in Zambia". Experimental and Applied Acarology 12: 9–26. doi:10.1007/BF01204396.
- ↑ Stachurski, F. (2000). "Invasion of West African cattle by the tick Amblyomma variegatum". Medical and Veterinary Entomology 14 (4): 391–399. doi:10.1046/j.1365-2915.2000.00246.x. PMID 11129703.
- ↑ Lancaster, J.L. (1986). Arthropods in livestock and poultry production. Chichester: Ellis Horwood Ltd. ISBN 0-85312-790-5.
- ↑ Gothe, R.; Kunze, K.; Hoogstraal, H. (1979). "The mechanisms of pathogenicity in the tick paralyses". Journal of Medical Entomology 16 (5): 357–369. PMID 232161.
- ↑ Stone, B.F. (1989). "Tick/host interactions for Ixodes holocyclus: role, effects, biosynthesis and nature of its allergenic oral secretions". Experimental and Applied Acarology 7: 59–69. doi:10.1007/BF01200453.
- ↑ Barker S.C. (2014) Ticks of Australia. The species that infest domestic animals and humans. Zootaxa, 3816, 1-144, http://mapress.com/zootaxa/2014/f/zt03816p144.pdf
- ↑ Martinez, D. (1993). "Epidemiological studies of dermatophilosis in the Caribbean". Revue d'Elevage et de Medicine Veterinaire des Pays Tropicaux 10: 323–327.
- ↑ Coetzer, J.A.W. (1994). Infectious diseases of livestock with special reference to southern Africa. Cape Town: Oxford University Press. ISBN 0-19-570506-8.
- ↑ Hoogstraal, H. (1979). "Ticks and spirochetes". Acta Tropica 36 (2): 133–136. PMID 41420.
- ↑ Uilenberg, G. (1984). "Heartwater in the Caribbean". Preventive Veterinary Medicine 2: 255–267. doi:10.1016/0167-5877(84)90068-0.
- ↑ Norval, R.A.I. (1992). The epidemiology of theileriosis in Africa. London: Academic Press. ISBN 0-12-521740-4.
- ↑ Katzer, F. (2010). "Genotypic diversity: a survival strategy for the apicomplexan parasite Theileria parva". Veterinary Parasitology 167 (2–4): 236–243. doi:10.1016/j.vetpar.2009.09.025. PMC 2817781. PMID 19837514.
- ↑ George, J.E.; Pound, J.M.; Davey, R.B. (2008). "Acaricides for controlling ticks on cattle and the problem of acaricide resistance". In: Ticks - biology, disease and control Eds. Bowman A.S. & Nuttall P.A.; Cambridge University Press: 408–423.
- ↑ Willadsen, P. (2006). "Tick control: thoughts on a research agenda" (PDF). Veterinary Parasitology 138: 161–168. doi:10.1016/j.vetpar.2006.01.050.
- ↑ Mount, G.A. (1991). "Computer simulation of Boophilus cattle tick population dynamics". Journal of Medical Entomology 28 (2): 223–240. PMID 2056504.
- ↑ Randolph, S.E.; Rodgers D.J. (1997). "A generic population model for the African tick Rhipicephalus appendiculatus". Parasitology 115 (3): 265–279. doi:10.1017/S0031182097001315. PMID 9300464.
- ↑ Schmidtmann, E.T. (1994). "Ecologically based strategies for controlling ticks. In: Ecological dynamics of tick-borne zoonoses Eds. Sonenshine D.E. & Mather T.N., Oxford University Press": 240–280.
- ↑ Hewetson, R.W. (1972). "The inheritance of resistance by cattle to cattle tick". Australian Veterinary Journal 48 (5): 299–303. doi:10.1111/j.1751-0813.1972.tb05161.x. PMID 5068812.
- ↑ Harley, K.L.S.; Wilkinson, P.R. (1971). "A modification of pasture spelling to reduce acaricide treatements for cattle tick control". Australian Veterinary Journal 47 (3): 108–111. doi:10.1111/j.1751-0813.1971.tb14750.x. PMID 5103591.
- ↑ Elder, J.K. (1980). "A survey concerning cattle tick control in Queensland, 4. Use of resistant cattle and pasture spelling". Australian Veterinary Journal 56 (5): 219–223. doi:10.1111/j.1751-0813.1980.tb15976.x. PMID 7436925.
- ↑ Timms, P. (1983). "Babesia bovis: comparison of culture-derived parasites, non-living antigen and conventional vaccine in the protection of cattle against heterologous challenge". Australian Veterinary Journal 60 (3): 75–77. doi:10.1111/j.1751-0813.1983.tb05874.x. PMID 6347164.
- ↑ Pipano, E. (1995). "Live vaccine against hemoparasitic disease in livestock". Veterinary Parasitology 57 (1–3): 213–231. doi:10.1016/0304-4017(94)03122-D. PMID 7597786.
- ↑ McKeever, D.J. (1999). "Protective immune mechanisms against Theileria parva: evolution of vaccine development strategies". Parasitology Today 15: 253–267. doi:10.1016/S0169-4758(99)01465-9.
- ↑ Willadsen, P. (1996). "Comparative vaccination of cattle against Boophilus microplus with recombinant antigen Bm86 alone or in combination with recombinant Bm91". Parasite Immunology 18 (5): 241–246. doi:10.1046/j.1365-3024.1996.d01-90.x. PMID 9229376.
External links
- Ticks. Centers for Disease Control and Prevention, USA.
- Tick-borne Livestock Diseases and their Vectors: Five-part Series. Food and Agriculture Organization of the United Nations.
- Colorado Ticks and Tick-Borne Diseases. Colorado State University Extension, updated 2013.
- Ticks. Urban Integrated Pest Management in the Southern Region.
- Ticks. Livestock Veterinary Entomology. Texas A&M AgriLife Extension.
- Paralysis Ticks. Australian Venom Research Unit.
- Prine, K. C. and A. C. Hodges. Tropical bont tick, Amblyomma variegatum. EENY-518. University of Florida IFAS. Published 2012, updated 2013.
Further reading
- Baker, A.S. (1999) Mites and Ticks of Domestic Animals: an identification guide and information source. London: The Stationery Office, ISBN 0-11-310049-3.
- Bowman, A. S. & Nuttall, P. A. (2008) Ticks: Biology, Disease and Control. Cambridge University Press, Cambridge, ISBN 978-0-521-86761-0
- Estrada-Peña, A., Bouattour, A., Camicas, J.-L. & Walker, A.R. (2004). Ticks of domestic animals in the Mediterranean Region. University of Zaragoza, ISBN 84-96214-18-4. https://www.researchgate.net/profile/Agustin_Estrada-Pena/publications/?page=5&sorting=newest
- Fivaz, B., Petney, T. & Horak I. (1992) Tick vector biology: medical and veterinary aspects. Springer-Verlag, Heidelberg, ISBN 3-540-54045-8
- Howell C.J., Walker J.B. & Nevill E.M. (1978) Ticks, mites and insects infesting domestic animals in South Africa. Republic of South Africa Department of Agricultural Technical Services, Science Bulletin 393, Pretoria.
- Latif, A.A. (2013) Illustrated guide to identification of African tick species. Agricultural Research Council, Pretoria. ISBN 978-0-9922220-5-5
- Roberts, F.H.S. (1970) Australian ticks. Commonwealth Scientific and Industrial Research Organisation, Melbourne.
- Slamon, M. & Tarrés-Call, J. (eds) (2013) Ticks and Tick-borne Diseases: Geographical Distribution and Control Strategies in the Euro-Asia Region. CABI, Wallingford. ISBN 978-1-84593-853-6.
- Sonenshine, D.E. & Mather, T.N. (eds) (1994) Ecological Dynamics of Tick-borne Zoonoses. Oxford University Press, New York. ISBN 0-19-507313-4
- Spickett, A.M. (2013) Ixodid ticks of major economic importance and their distribution in South Africa. Agricultural Research Council, Pretoria. ISBN 978-0-9922220-4-8
- Walker, A.R., Bouattour, A., Camicas, J.-L., Estrada-Peña, A., Horak, I.G., Latif, A.A., Pegram, R.G. & Preston, P.M. 2003. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports, Edinburgh. ISBN 0-9545173-0-X, http://www.alanrwalker.com/index/guidebooks