Tiopronin
 | |
| Names | |
|---|---|
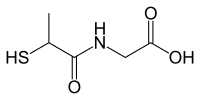
| IUPAC name
2-(2-sulfanylpropanoylamino)acetic acid | |
| Other names
2-mercaptopropionylglycine Acadione Thiola | |
| Identifiers | |
| 1953-02-2 29335-92-0 R | |
| 1859822 | |
| ChEMBL | ChEMBL1314 |
| ChemSpider | 5283 180938 R 643292 S |
| EC Number | 217-778-4 |
| Jmol interactive 3D | Image Image |
| KEGG | D01430 |
| MeSH | Tiopronin |
| PubChem | 5483 208825 R 736152 S |
| RTECS number | MC0596500 |
| UNII | C5W04GO61S |
| |
| |
| Properties | |
| C5H9NO3S | |
| Molar mass | 163.19 g·mol−1 |
| Appearance | White, opaque crystals |
| Melting point | 93 to 98 °C (199 to 208 °F; 366 to 371 K) |
| log P | −0.674 |
| Acidity (pKa) | 3.356 |
| Basicity (pKb) | 10.641 |
| Pharmacology | |
| ATC code | R05 QG04BC90 |
| Legal status |
|
| |
| Hazards | |
| GHS pictograms |  |
| GHS signal word | WARNING |
| H302 | |
| EU classification (DSD) |
|
| R-phrases | R22 |
| S-phrases | S36/37 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
1,300 mg kg−1 (oral, rat) |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tiopronin (trade name Thiola) is a prescription thiol drug used to control the rate of cystine precipitation and excretion in the disease cystinuria.[1][2] Due to the rarity of the disorder, tiopronin falls under the classification of an orphan drug. It is somewhat similar to penicillamine in both chemistry and pharmacology.
Uses
Tiopronin is used primarily for cystinuria and is well known in the cystinuric community. Depending on the severity of a person's cystinuria, tiopronin may be taken for life, possibly starting in early childhood. The drug works by reacting with urinary cysteine to form a more soluble, disulfide linked, tiopronin-cysteine complex.[3]
It may also be used for Wilson's disease (an overload of copper in the body), and has also been investigated for the treatment of arthritis,[4][5] though tiopronin is not an anti-inflammatory.
Tiopronin is also sometimes used as a stabilizing agent for metal nanoparticles. The thiol group binds to the nanoparticles, preventing coagulation.[6]
Side effects
Tiopronin may present a variety of side effects, which are broadly similar to those of D-penicillamine and other compounds containing active sulfhydryl groups.[7] Its pharmokinetics have been studied.[3]
Costs
In the U.S., the drug was marketed by Mission Pharmacal at $1.50 per pill, but the rights were bought by Retrophin, owned by Martin Shkreli, and the price increased to $30 per pill.[8][9][10] A potential alternative in treatment and costs has arisen with the drug 'Bucillamine'. [11]
References
- ↑ Lindell, Å.; Denneberg, T.; Hellgren, E.; Jeppsson, J. -O.; Tiselius, H. -G. "Clinical course and cystine stone formation during tiopronin treatment". Urological Research 23 (2): 111–117. doi:10.1007/BF00307941.
- ↑ Coe, Fredric L.; Parks, Joan H.; Asplin, John R. (15 October 1992). "The Pathogenesis and Treatment of Kidney Stones". New England Journal of Medicine 327 (16): 1141–1152. doi:10.1056/NEJM199210153271607.
- 1 2 Carlsson, M. S.; Denneberg, T.; Emanuelsson, B.-M.; K�gedal, B.; Lindgren, S. (August 1993). "Pharmacokinetics of oral tiopronin". European Journal of Clinical Pharmacology 45 (1): 79–84. doi:10.1007/BF00315354. replacement character in
|last4=at position 2 (help) - ↑ Delecoeuillerie, G (30 April 1989). "[Tolerability and therapeutic maintenance of tiopronin, new basic treatment of rheumatoid arthritis. Apropos of long-term follow-up of 268 cases].". Revue du rhumatisme et des maladies osteo-articulaires 56 (5 Pt 2): 38–42. PMID 2740804.
- ↑ Pasero, Giampiero; Pellegrini, Pietro; Ambanelli, Umberto; Ciompi, Maria Laura; Colamussi, Vincenzo; Ferraccioli, Gianfranco; Barbieri, Paola; Mazzoni, Maria Rosa; Menegale, Germano; Trippi, Donatella (August 1982). "Controlled multicenter trial of tiopronin and D-penicillamine for rheumatoid arthritis". Arthritis & Rheumatism 25 (8): 923–929. doi:10.1002/art.1780250803.
- ↑ Jennifer A. Dahl, Bettye L. S. Maddux, and James E. Hutchison (2007). "Toward Greener Nanosynthesis". Chemical Reviews 107 (6): 2228–2269. doi:10.1021/cr050943k. PMID 17564480.
- ↑ Jaffe, Israeli A. (March 1986). "Adverse effects profile of sulfhydryl compounds in man". The American Journal of Medicine 80 (3): 471–476. doi:10.1016/0002-9343(86)90722-9.
- ↑ "The huge price hike as sales strategy, taken to extremes by Retrophin". FiercePharma.
- ↑ "Why would Martin Shkreli hike an old drug price by 5000%? Only a 'moron' would ask". FierceBiotech.
- ↑ "‘Pharma bro’ Martin Shkreli gouged kids with kidney disease before ripping off AIDS patients". rawstory.com.
- ↑ Market Wired Bucillamine article
External links
| ||||||||||||||||||||