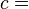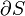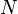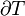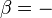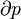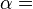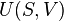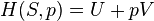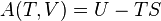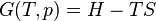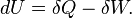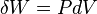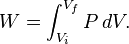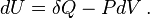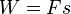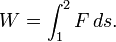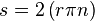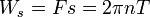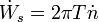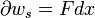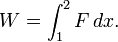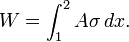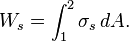Work (thermodynamics)
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
In thermodynamics, work performed by a system is the energy transferred by the system to its surroundings, that is fully accounted for solely by macroscopic forces exerted on the system by factors external to it, that is to say, factors in its surroundings. Thermodynamic work is a version of the concept of work in physics.
The external factors may be electromagnetic,[1][2][3] gravitational,[4] or pressure/volume or other simply mechanical constraints.[5] Thermodynamic work is defined to be measurable solely from knowledge of such external macroscopic forces. These forces are associated with macroscopic state variables of the system that always occur in conjugate pairs, for example pressure and volume,[5] magnetic flux density and magnetization.[2] In the SI system of measurement, work is measured in joules (symbol: J). The rate at which work is performed is power.
History
1824
Work, i.e. "weight lifted through a height", was originally defined in 1824 by Sadi Carnot in his famous paper Reflections on the Motive Power of Fire, he used the term motive power. Specifically, according to Carnot:
- We use here motive power to express the useful effect that a motor is capable of producing. This effect can always be likened to the elevation of a weight to a certain height. It has, as we know, as a measure, the product of the weight multiplied by the height to which it is raised.
1845
.png)
In 1845, the English physicist James Joule wrote a paper On the mechanical equivalent of heat for the British Association meeting in Cambridge.[6] In this paper, he reported his best-known experiment, in which the mechanical power released through the action of a "weight falling through a height" was used to turn a paddle-wheel in an insulated barrel of water.
In this experiment, the friction and agitation of the paddle-wheel on the body of water caused heat to be generated which, in turn, increased the temperature of water. Both the temperature change ∆T of the water and the height of the fall ∆h of the weight mg were recorded. Using these values, Joule was able to determine the mechanical equivalent of heat. Joule estimated a mechanical equivalent of heat to be 819 ft•lbf/Btu (4.41 J/cal). The modern day definitions of heat, work, temperature, and energy all have connection to this experiment.
Overview
Mechanical thermodynamic work is performed by actions such as compression, and including shaft work, stirring, and rubbing. In the simplest cases, for example, there are work of change of volume against a resisting pressure, and work without change of volume, known as isochoric work. An example of isochoric work is when an outside agency, in the surrounds of the system, drives a frictional action on the surface of the system. In this case the dissipation is usually not confined to the system, and the quantity of energy so transferred as work must be estimated through the overall change of state of the system as measured by both its mechanically and externally measurable deformation variables (such as its volume), and its corresponding non-deformation variable (such as its pressure). In a process of transfer of energy as work, the change of internal energy of the system is then defined in theory by the amount of adiabatic work that would have been necessary to reach the final from the initial state, such adiabatic work being measurable only through the externally measurable mechanical or deformation variables of the system, that provide full information about the forces exerted by the surroundings on the system during the process. In the case of some of Joule's measurements, the process was so arranged that heat produced outside the system (in the paddles) by the frictional process was practically entirely transferred into the system during the process, so that the quantity of work done by the surrounds on the system could be calculated as shaft work, an external mechanical variable.[7][8]
The amount of energy transferred as work is measured through quantities defined externally to the system of interest, and thus belonging to its surroundings. In an important sign convention, work that adds to the internal energy of the system is counted as positive. Nevertheless, on the other hand, for historical reasons, an oft-encountered sign convention is to consider work done by the system on its surroundings as positive. Although all real physical processes entail some dissipation of kinetic energy, it is a matter of definition in thermodynamics that the dissipation that results from transfer of energy as work occurs only inside the system. Energy dissipated outside the system, in the process of transfer of energy, is not counted as thermodynamic work, because it is not fully accounted for by macroscopic forces exerted on the system by external factors. Thermodynamic work does not account for any energy transferred between systems as heat or though transfer of matter.
All the various mechanical and non-mechanical forms of work can be converted into each other with no fundamental limitation due to the laws of thermodynamics, so that the energy conversion efficiency can approach 100% in some cases.[9] In particular, all forms of work can be converted into the mechanical work of lifting a weight, which was the original form of thermodynamic work considered by Carnot and Joule (see History section above). Some authors have considered this equivalence to the lifting of a weight as a defining characteristic of work.[10][11][12][13] In contrast, the conversion of heat into work in a heat engine can never exceed the Carnot efficiency, as a consequence of the second law of thermodynamics.
For a closed thermodynamic system, the first law of thermodynamics relates changes in the internal energy to two forms of energy transfer, as heat and as work. In theory, heat is properly defined for a process in a closed system (no transfer of matter) by the amount of adiabatic work that would be needed to effect the change occasioned by the process. In practice it is often estimated calorimetrically, through change of temperature of a known quantity of calorimetric material substance; it is of the essence of heat transfer that it is not mediated by the externally defined forces variables that define work. This distinction between work and heat is essential to thermodynamics.
Beyond the conceptual scope of thermodynamics proper, heat is transferred by the microscopic thermal motions of particles and their associated inter-molecular potential energies,[14] or by radiation.[15][16] There are two forms of macroscopic heat transfer by direct contact between a closed system and its surroundings: conduction,[17] and radiation. There are several forms of dissipative transduction of energy that can occur internally within a system at a microscopic level, such as friction including bulk and shear viscosity,[18] chemical reaction,[1] unconstrained expansion as in Joule expansion and in diffusion, and phase change;[1] these are not transfers of heat between systems.
Convection of internal energy is a form a transport of energy but is in general not, as sometimes mistakenly supposed (a relic of the caloric theory of heat), a form of transfer of energy as heat, because convection is not in itself a microscopic motion of microscopic particles or their intermolecular potential energies, or photons; nor is it a of transfer of energy as work. Nevertheless, if the wall between the system and its surroundings is thick and contains fluid, in the presence of a gravitational field, convective circulation within the wall can be considered as indirectly mediating transfer of energy as heat between the system and its surroundings, though they are not in direct contact.
For an open system, the first law of thermodynamics admits three forms of energy transfer, as work, as heat, and as energy associated with matter that is transferred. The latter cannot be split uniquely into heat and work components.
Formal definition
In thermodynamics, the quantity of work done by a closed system on its surroundings is defined by factors strictly confined to the interface of the surroundings with the system and to the surroundings of the system, for example an extended gravitational field in which the system sits, that is to say, to things external to the system. There are a few especially important kinds of thermodynamic work.
A simple example of one of those important kinds is pressure-volume work. The pressure of concern is that exerted by the surroundings on the surface of the system, and the volume of interest is the negative of the increment of volume gained by the system from the surroundings. It is usually arranged that the pressure exerted by the surroundings on the surface of the system is well defined and equal to the pressure exerted by the system on the surroundings. This arrangement for transfer of energy as work can be varied in a particular way that depends on the strictly mechanical nature of pressure-volume work. The variation consists in letting the coupling between the system and surroundings be through a rigid rod that links pistons of different areas for the system and surroundings. Then for a given amount of work transferred, the exchange of volumes involves different pressures, inversely with the piston areas, for mechanical equilibrium. This cannot be done for the transfer of energy as heat because of its non-mechanical nature.[19]
Another important kind of work is isochoric work, that is to say work that involves no eventual overall change of volume of the system between the initial and the final states of the process. Examples are friction on the surface of the system as in Rumford's experiment; shaft work such as in Joule's experiments; and slow vibrational action on the system that leaves its eventual volume unchanged, but involves friction within the system. Isochoric work for a body in its own state of internal thermodynamic equilibrium is done only by the surroundings on the body, not by the body on the surroundings, so that the sign of isochoric work with the present sign convention is always negative.
When work is done by a closed system that cannot pass heat in or out because it is adiabatically isolated, the work is referred to as being adiabatic in character. Adiabatic work can be of the pressure-volume kind or of the isochoric kind, or both.
According to the first law of thermodynamics for a closed system, any net increase in the internal energy U must be fully accounted for, in terms of heat δQ entering the system and the work δW done by the system:[14]
The letter d indicates an exact differential, expressing that internal energy U is a property of the state of the system; they depend only on the original state and the final state, and not upon the path taken. In contrast, the Greek deltas (δ's) in this equation reflect the fact that the heat transfer and the work transfer are not properties of the final state of the system. Given only the initial state and the final state of the system, one can only say what the total change in internal energy was, not how much of the energy went out as heat, and how much as work. This can be summarized by saying that heat and work are not state functions of the system.[14]
The minus sign in front of 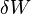 indicates that a positive amount of work done by the system leads to energy being lost from the system. This is the sign convention for work in many textbooks on physics. This sign convention entails that a non-zero quantity of isochoric work always has a negative sign, because of the second law of thermodynamics. This is the sign convention used in the present article except where otherwise noted.
indicates that a positive amount of work done by the system leads to energy being lost from the system. This is the sign convention for work in many textbooks on physics. This sign convention entails that a non-zero quantity of isochoric work always has a negative sign, because of the second law of thermodynamics. This is the sign convention used in the present article except where otherwise noted.
An alternate sign convention is to consider the work performed on the system by its surroundings as positive. This leads to a change in sign of the work, so that  . This is the convention adopted by many modern textbooks.[21][22][23]
. This is the convention adopted by many modern textbooks.[21][22][23]
Pressure-volume work
Pressure-volume work (or PV work) occurs when the volume V of a system changes. PV work is often measured in units of litre-atmospheres where 1L·atm = 101.325J. However, the litre-atmosphere is not a recognised unit in the SI system of units, which measures P in Pascal (Pa), V in m3, and PV in Joule (J), where 1 J = 1 Pa·m3. PV work is an important topic in chemical thermodynamics.
For a process in a closed system, occurring slowly enough for accurate definition of the pressure on the inside of the system's wall that moves and transmits force to the surroundings, described as quasi-static,[24][25] work is represented by the following equation between differentials:
where
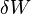 denotes an infinitesimal increment of work done by the system, transferring energy to the surroundings;
denotes an infinitesimal increment of work done by the system, transferring energy to the surroundings;
 denotes the pressure inside the system, that it exerts on the moving wall that transmits force to the surroundings.[26] In the alternate sign convention the right hand side has a negative sign.[23]
denotes the pressure inside the system, that it exerts on the moving wall that transmits force to the surroundings.[26] In the alternate sign convention the right hand side has a negative sign.[23]
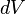 denotes the infinitesimal increment of the volume of the system.
denotes the infinitesimal increment of the volume of the system.
Moreover,
where
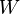 denotes the work done by the system during the whole of the reversible process.
denotes the work done by the system during the whole of the reversible process.
The first law of thermodynamics can then be expressed as
(In the alternate sign convention where W = work done on the system, 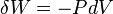 . However,
. However, 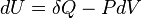 is unchanged.)
is unchanged.)
Path dependence
As for all kinds of work, in general PV work is path-dependent and is therefore a thermodynamic process function. In general, the term P dV is not an exact differential.[27] The statement that a process is reversible and adiabatic gives important information about the process, but does not determine the path uniquely, because the path can include several slow goings backward and forward in volume, as long as there is no transfer of energy as heat. The first law of thermodynamics states 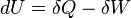 . For an adiabatic process,
. For an adiabatic process, 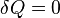 and thus the integral amount work done is equal to minus the change in internal energy. For a reversible adiabatic process, the integral amount of work done during the process depends only on the initial and final states of the process, and is the one and the same for every intermediate path.
and thus the integral amount work done is equal to minus the change in internal energy. For a reversible adiabatic process, the integral amount of work done during the process depends only on the initial and final states of the process, and is the one and the same for every intermediate path.
If the process took a path other than an adiabatic path, the work would be different. This would only be possible if heat flowed into/out of the system. In a non-adiabatic process, there are indefinitely many paths between the initial and final states.
In the current mathematical notation, the differential 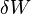 is an inexact differential.[14]
is an inexact differential.[14]
In another notation, δW is written đW (with a line through the d). This notation indicates that đW is not an exact one-form. The line-through is merely a flag to warn us there is actually no function (0-form) W which is the potential of đW. If there were, indeed, this function W, we should be able to just use Stokes Theorem to evaluate this putative function, the potential of đW, at the boundary of the path, that is, the initial and final points, and therefore the work would be a state function. This impossibility is consistent with the fact that it does not make sense to refer to the work on a point in the PV diagram; work presupposes a path.
Other mechanical forms of work
There are several ways of doing work, each in some way related to a force acting through a distance.[28] In basic mechanics,the work done by a constant force F on a body displaced a distance s in the direction of the force is given by
If the force is not constant, the work done is obtained by integrating the differential amount of work,
Shaft work
Energy transmission with a rotating shaft is very common in engineering practice. Often the torque T applied to the shaft is constant which means that the force F applied is constant. For a specified constant torque, the work done during n revolutions is determined as follows: A force F acting through a moment arm r generates a torque T
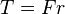 →
→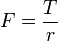
This force acts through a distance s, which is related to the radius r by
The shaft work is then determined from:
The power transmitted through the shaft is the shaft work done per unit time, which is expressed as
Spring work
When a force is applied on a spring, and the length of the spring changes by a differential amount dx, the work done is
For linear elastic springs, the displacement x is proportional to the force applied
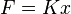 ,
,
where K is the spring constant and has the unit of N/m. The displacement x is measured from the undisturbed position of the spring (that is, X=0 when F=0). Substituting the two equations
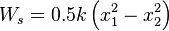 ,
,
where x1 and x2 are the initial and the final displacement of the spring respectively, measured from the undisturbed position of the spring.
Work done on elastic solid bars
Solids are often modeled as linear springs because under the action of a force they contract or elongate, and when the force is lifted, they return to their original lengths, like a spring. This is true as long as the force is in the elastic range, that is, not large enough to cause permanent or plastic deformation. Therefore, the equations given for a linear spring can also be used for elastic solid bars. Alternately, we can determine the work associated with the expansion or contraction of an elastic solid bar by replacing the pressure P by its counterpart in solids, normal stress σ=F/A in the work expansion
where A is the cross sectional area of the bar.
Work associated with the stretching of liquid film
Consider a liquid film such as a soap film suspended on a wire frame. Some force is required to stretch this film by the movable portion of the wire frame. This force is used to overcome the microscopic forces between molecules at the liquid-air interface. These microscopic forces are perpendicular to any line in the surface and the force generated by these forces per unit length is called the surface tension σ whose unit is N/m. Therefore, the work associated with the stretching of a film is called surface tension work, and is determined from
where dA=2b dx is the change in the surface area of the film. The factor 2 is due to the fact that the film has two surfaces in contact with air. The force acting on the moveable wire as a result of surface tension effects is F=2b σ, where σ is the surface tension force per unit length.
Free energy and exergy
The amount of useful work which may be extracted from a thermodynamic system is determined by the second law of thermodynamics. Under many practical situations this can be represented by the thermodynamic availability, or Exergy, function. Two important cases are: in thermodynamic systems where the temperature and volume are held constant, the measure of useful work attainable is the Helmholtz free energy function; and in systems where the temperature and pressure are held constant, the measure of useful work attainable is the Gibbs free energy.
Non-mechanical forms of work
Non-mechanical work in thermodynamics is work determined by long-range forces penetrating into the system as force fields. The action of such forces can be initiated by events in the surroundings of the system, or by thermodynamic operations on the shielding walls of the system. The long-range forces are forces in the ordinary physical sense of the word, not the so-called 'thermodynamic forces' of non-equilibrium thermodynamic terminology.
The non-mechanical work of long-range forces can have either positive or negative sign, work being done by the system on the surroundings, or vice versa. Work done by long-range forces can be done indefinitely slowly, so as to approach the fictive reversible quasi-static ideal, in which entropy is not created in the system by the process.
In thermodynamics, non-mechanical work is to be contrasted with mechanical work that is done by forces in immediate contact between the system and its surroundings. If the putative 'work' of a process cannot be defined as either long-range work or else as contact work, then sometimes it cannot be described by the thermodynamic formalism as work at all. Nevertheless, the thermodynamic formalism allows that energy can be transferred between an open system and its surroundings by processes for which work is not defined. An example is when the wall between the system and its surrounds is not considered as idealized and vanishingly thin, so that processes can occur within the wall, such as friction affecting the transfer of matter across the wall; in this case, the forces of transfer are neither strictly long-range nor strictly due to contact between the system and its surrounds; the transfer of energy can then be considered as by convection, and assessed in sum just as transfer of internal energy. This is conceptually different from transfer of energy as heat through a thick fluid-filled wall in the presence of a gravitational field, between a closed system and its surroundings; in this case there may convective circulation within the wall but the process may still be considered as transfer of energy as heat between the system and its surroundings; if the whole wall is moved by the application of force from the surroundings, without change of volume of the wall, so as to change the volume of the system, then it is also at the same time transferring energy as work. A chemical reaction within a system can lead to electrical long-range forces and to electric current flow, which transfer energy as work between system and surroundings, though the system's chemical reactions themselves (except for the special limiting case in which in they are driven through devices in the surroundings so as to occur along a line of thermodynamic equilibrium) are always irreversible and do not directly interact with the surroundings of the system.[29]
Non-mechanical work contrasts with pressure-volume work. Pressure-volume work is one of the two mainly considered kinds of mechanical contact work. A force acts on the interfacing wall between system and surroundings. The force is that due to the pressure exerted on the interfacing wall by the material inside the system; that pressure is an internal state variable of the system, but is properly measured by external devices at the wall. The work is due to change of system volume by expansion or contraction of the system. If the system expands, in the present article it is said to do positive work on the surroundings. If the system contracts, in the present article it is said to do negative work on the surroundings. Pressure-volume work is a kind of contact work, because it occurs through direct material contact with the surrounding wall or matter at the boundary of the system. It is accurately described by changes in state variables of the system, such as the time courses of changes in the pressure and volume of the system. The volume of the system is classified as a "deformation variable", and is properly measured externally to the system, in the surroundings. Pressure-volume work can have either positive or negative sign. Pressure-volume work, performed slowly enough, can be made to approach the fictive reversible quasi-static ideal.
Non-mechanical work also contrasts with shaft work. Shaft work is the other of the two mainly considered kinds of mechanical contact work. It transfers energy by rotation, but it does not eventually change the shape or volume of the system. Because it does not change the volume of the system it is not measured as pressure-volume work, and it is called isochoric work. Considered solely in terms of the eventual difference between initial and final shapes and volumes of the system, shaft work does not make a change. During the process of shaft work, for example the rotation of a paddle, the shape of the system changes cyclically, but this does not make an eventual change in the shape or volume of the system. Shaft work is a kind of contact work, because it occurs through direct material contact with the surrounding matter at the boundary of the system. A system that is initially in a state of thermodynamic equilibrium cannot initiate any change in its internal energy. In particular, it cannot initiate shaft work. This explains the curious use of the phrase "inanimate material agency" by Kelvin in one of his statements of the second law of thermodynamics. Thermodynamic operations or changes in the surroundings are considered to be able to create elaborate changes such as indefinitely prolonged, varied, or ceased rotation of a driving shaft, while a system that starts in a state of thermodynamic equilibrium is inanimate and cannot spontaneously do that.[30] Thus the sign of shaft work is always negative, work being done on the system by the surroundings. Shaft work can hardly be done indefinitely slowly; consequently it always produces entropy within the system, because it relies on friction or viscosity within the system for its transfer.[31] The foregoing comments about shaft work apply only when one ignores that the system can store angular momentum and its related energy.
Examples of non-mechanical work modes include
- Electrical work – where the force is defined by the surroundings' voltage (the electrical potential) and the generalized displacement is change of spatial distribution of electrical charge
- Magnetic work – where the force is defined by the surroundings' magnetic field strength and the generalized displacement is change of total magnetic dipole moment
- Electrical polarization work – where the force is defined by the surroundings' electric field strength and the generalized displacement is change of the polarization of the medium (the sum of the electric dipole moments of the molecules)
- Gravitational work – where the force is defined by the surroundings' gravitational field and the generalized displacement is change of the spatial distribution of the matter within the system.
See also
- Electrical work
- Chemical reactions
- Microstate (statistical mechanics) - includes Microscopic definition of work
References
- 1 2 3 Guggenheim, E.A. (1985). Thermodynamics. An Advanced Treatment for Chemists and Physicists, seventh edition, North Holland, Amsterdam, ISBN 0444869514.
- 1 2 Jackson, J.D. (1975). Classical Electrodynamics, second edition, John Wiley and Sons, New York, ISBN 978-0-471-43132-9.
- ↑ Konopinski, E.J. (1981). Electromagnetic Fields and Relativistic Particles, McGraw-Hill, New York, ISBN 007035264X.
- ↑ North, G.R., Erukhimova, T.L. (2009). Atmospheric Thermodynamics. Elementary Physics and Chemistry, Cambridge University Press, Cambridge (UK), ISBN 9780521899635.
- 1 2 Kittel, C. Kroemer, H. (1980). Thermal Physics, second edition, W.H. Freeman, San Francisco, ISBN 0716710889.
- ↑ Joule, J.P. (1845) "On the Mechanical Equivalent of Heat", Brit. Assoc. Rep., trans. Chemical Sect, p.31, which was read before the British Association at Cambridge, June
- ↑ Buchdahl, H.A. (1966). The Concepts of Classical Thermodynamics, Cambridge University Press, London, p. 40.
- ↑ Bailyn, M. (1994). A Survey of Thermodynamics, American Institute of Physics Press, New York, ISBN 0-88318-797-3, pp. 35–36.
- ↑ F.C.Andrews Thermodynamics: Principles and Applications (Wiley-Interscience 1971), ISBN 0-471-03183-6, p.17-18.
- ↑ Silbey, R.J., Alberty, R.A., Bawendi, M.G. (2005). Physical Chemistry, 4th edition, Wiley, Hoboken NJ., ISBN 978-0-471-65802-3, p.31
- ↑ K.Denbigh The Principles of Chemical Equilibrium (Cambridge University Press 1st ed. 1955, reprinted 1964), p.14.
- ↑ J.Kestin A Course in Thermodynamics (Blaisdell Publishing 1966), p.121.
- ↑ M.A.Saad Thermodynamics for Engineers (Prentice-Hall 1966) p.45-46.
- 1 2 3 4 5 G.J. Van Wylen and R.E. Sonntag, Fundamentals of Classical Thermodynamics, Chapter 4 - Work and heat, (3rd edition)
- ↑ Prevost, P. (1791). Mémoire sur l'equilibre du feu. Journal de Physique (Paris), vol 38 pp. 314-322.
- ↑ Planck, M. (1914). The Theory of Heat Radiation, second edition translated by M. Masius, P. Blakiston's Son and Co., Philadelphia, 1914.
- ↑ Kondepudi, D. (2008). Introduction to Modern Thermodynamics, John Wiley and Sons, Chichester, ISBN 978-0-470-01598-8.
- ↑ Rayleigh, J.W.S (1878/1896/1945). The Theory of Sound, volume 2, Dover, New York,
- ↑ Tisza, L. (1966). Generalized Thermodynamics, M.I.T. Press, Cambridge MA, p. 37.
- ↑ Freedman, Roger A., and Young, Hugh D. (2008). 12th Edition. Chapter 19: First Law of Thermodynamics, page 656. Pearson Addison-Wesley, San Francisco.
- ↑ Quantities, Units and Symbols in Physical Chemistry (IUPAC Green Book) See Sec. 2.11 Chemical Thermodynamics, p. 56.
- ↑ Planck, M. (1897/1903). Treatise on Thermodynamics, translated by A. Ogg, Longmans, Green & Co., London., p. 43.
- 1 2 Adkins, C.J. (1968/1983). Equilibrium Thermodynamics, (1st edition 1968), third edition 1983, Cambridge University Press, Cambridge UK, ISBN 0-521-25445-0, pp. 35–36.
- ↑ Callen, H. B. (1960/1985), Thermodynamics and an Introduction to Thermostatistics, (first edition 1960), second edition 1985, John Wiley & Sons, New York, ISBN 0–471–86256–8, p. 19.
- ↑ Münster, A. (1970), Classical Thermodynamics, translated by E. S. Halberstadt, Wiley–Interscience, London, ISBN 0-471-62430-6, p. 24.
- ↑ Borgnakke, C., Sontag, R.E. (2009). Fundamentals of Thermodynamics, seventh edition, Wiley, ISBN 978-0-470-04192-5, p. 94.
- ↑ Haase, R. (1971). Survey of Fundamental Laws, chapter 1 of Thermodynamics, pages 1–97 of volume 1, ed. W. Jost, of Physical Chemistry. An Advanced Treatise, ed. H. Eyring, D. Henderson, W. Jost, Academic Press, New York, lcn 73–117081, p. 21.
- ↑ Yunus A. Cengel and Michael A. Boles,Thermodynamics: An Engineering Approach 7th Edition, , McGraw-Hill, 2010,ISBN 007-352932-X
- ↑ Prigogine, I., Defay, R. (1954). Chemical Thermodynamics, translation by D.H. Everett of the 1950 edition of Thermodynamique Chimique, Longmans, Green & Co., London, p. 43.
- ↑ Thomson, W. (March 1851). "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule’s equivalent of a Thermal Unit, and M. Regnault’s Observations on Steam". Transactions of the Royal Society of Edinburgh XX (part II): 261–268; 289–298. Also published in Thomson, W. (December 1852). "On the Dynamical Theory of Heat, with numerical results deduced from Mr Joule’s equivalent of a Thermal Unit, and M. Regnault’s Observations on Steam". Philos. Mag. 4 IV (22): 8–21. Retrieved 25 June 2012.
- ↑ Münster, A. (1970), Classical Thermodynamics, translated by E.S. Halberstadt, Wiley–Interscience, London, ISBN 0-471-62430-6, p. 45.