The Hallmarks of Cancer
"The Hallmarks of Cancer"[1] is a seminal[2][3] peer-reviewed article published in the journal Cell in January 2000 by the cancer researchers Douglas Hanahan and Robert Weinberg.
The authors believe that the complexity of cancer can be reduced to a small number of underlying principles. The paper argues that all cancers share six common traits ("hallmarks") that govern the transformation of normal cells to cancer (malignant or tumor) cells.
The traits ("hallmarks") that the authors highlight in the paper are (1) Cancer cells stimulate their own growth (Self-sufficiency in growth signals); (2) They resist inhibitory signals that might otherwise stop their growth (Insensitivity to anti-growth signals); (3) They resist their programmed cell death (Evading apoptosis); (4) They can multiply indefinitely (Limitless replicative potential) (5) They stimulate the growth of blood vessels to supply nutrients to tumors (Sustained angiogenesis); (6) They invade local tissue and spread to distant sites (Tissue invasion and metastasis).
By November 2010, the paper had been referenced over 15,000 times by other research papers, and was downloaded 20,000 times a year between 2004 and 2007.[4] As of March 2011, it was Cell's most cited article.[2]
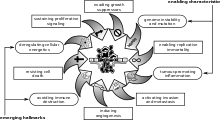
In an update published in 2011 ("Hallmarks of cancer: the next generation"), Weinberg and Hanahan proposed two new hallmarks: (1) abnormal metabolic pathways; and (2) evading the immune system.[5]
List of hallmarks

Cancer cells have defects in the control mechanisms that govern how often they divide, and in the feedback systems that regulate these control mechanisms (i.e. defects in homeostasis).
Normal cells grow and divide, but have many controls on that growth. They only grow when stimulated by growth factors. If they are damaged, a molecular brake stops them from dividing until they are repaired. If they can't be repaired, they commit cell suicide (apoptosis). They can only divide a limited number of times. They are part of a tissue structure, and remain where they belong. They need a blood supply to grow.
All these mechanisms must be overcome in order for a cell to develop into a cancer. Each mechanism is controlled by several proteins. A critical protein must malfunction in each of those mechanisms. These proteins become non-functional or malfunctioning when the DNA sequence of their genes is damaged through acquired or somatic mutations (mutations that are not inherited but occur after conception). This occurs in a series of steps, which Hanahan and Weinberg refer to as hallmarks.
| Capability | Simple analogy |
|---|---|
| Self-sufficiency in growth signals | "accelerator pedal stuck on" |
| Insensitivity to anti-growth signals | "brakes don't work" |
| Evading apoptosis | won't die when the body normally would kill the defective cell |
| Limitless replicative potential | infinite generations of descendants |
| Sustained angiogenesis | telling the body to give it a blood supply |
| Tissue invasion and metastasis | migrating and spreading to other organs and tissues |
Self-sufficiency in growth signals
- Cancer cells do not need stimulation from external signals (in the form of growth factors) to multiply.
Typically, cells of the body require hormones and other molecules that act as signals for them to grow and divide. Cancer cells, however, have the ability to grow without these external signals. There are multiple ways in which cancer cells can do this: by producing these signals themselves, known as autocrine signalling; by permanently activating the signalling pathways that respond to these signals; or by destroying 'off switches' that prevents excessive growth from these signals (negative feedback). In addition, cell division in normal, non-cancerous cells is tightly controlled. In cancer cells, these processes are deregulated because the proteins that control them are altered, leading to increased growth and cell division within the tumor.[7][8]
Insensitivity to anti-growth signals
- Cancer cells are generally resistant to growth-preventing signals from their neighbours.

To tightly control cell division, cells have processes within them that prevent cell growth and division. These processes are orchestrated by proteins known as tumor supressor genes. These genes take information from the cell to ensure that it is ready to divide, and will halt division if not (when the DNA is damaged, for example). In cancer, these tumour suppressor proteins are altered so that they don't effectively prevent cell division, even when the cell has severe abnormalities. Another way cells prevent over-division is that normal cells will also stop dividing when the cells fill up the space they are in and touch other cells; known as contact inhibition. Cancer cells do not have contact inhibition, and so will continue to grow and divide, regardless of their surroundings.[7][9]
Evading progammed cell death
- Apoptosis is a form of programmed cell death (cell suicide), the mechanism by which cells are programmed to die in the event they become damaged. Cancer cells are characteristically able to bypass this mechanism.
Cells have the ability to 'self-destruct'; a process known as apoptosis. This is required for organisms to grow and develop properly, for maintaining tissues of the body, and is also initiated when a cell is damaged or infected. Cancer cells, however, lose this ability; even though cells may become grossly abnormal, they do not apoptose. The cancer cells may do this by altering the mechanisms that detect the damage or abnormalities. This means that proper signalling cannot occur, thus apoptosis cannot activate. They may also have defects in the downstream signalling itself, or the proteins involved in apoptosis, each of which will also prevent proper apoptosis.[7][10]
Limitless replicative potential
- Non-cancer cells die after a certain number of divisions. Cancer cells escape this limit and are apparently capable of indefinite growth and division (immortality). But those immortal cells have damaged chromosomes, which can become cancerous.
Cells of the body don't normally have the ability to divide indefinitely. They have a limited number of divisions before the cells become unable to divide (senescence), or die (crisis). The cause of these barriers is primarily due to the DNA at the end of chromosomes, known as telomeres. Telomeric DNA shortens with every cell division, until it becomes so short it activates senescence, so the cell stops dividing. Cancer cells bypass this barrier by manipulating enzymes that increase the length of telomeres. Thus, they can divide indefinitely, without initiating senescence.[7][11]
Mammalian cells have an intrinsic program, the Hayflick limit, that limits their multiplication to about 60–70 doublings, at which point they reach a stage of senescence.
This limit can be overcome by disabling their pRB and p53 tumor suppressor proteins, which allows them to continue doubling until they reach a stage called crisis, with apoptosis, karyotypic disarray, and the occasional (10−7) emergence of an immortalized cell that can double without limit. Most tumor cells are immortalized.
The counting device for cell doublings is the telomere, which decreases in size (loses nucleotides at the ends of chromosomes) during each cell cycle. Many cancers involve the upregulation of telomerase, the enzyme that maintains telomeres.
Developing blood vessels
- Angiogenesis is the process by which new blood vessels are formed. Cancer cells appear to be able to kickstart this process, ensuring that such cells receive a continual supply of oxygen and other nutrients.
Normal tissues of the body have blood vessels running through them that deliver oxygen from the lungs. Cells must be close to the blood vessels to get enough oxygen for them to survive. New blood vessels are formed during the development of embryos, during wound repair and during the female reproductive cycle. An expanding tumour requires new blood vessels to deliver adequate oxygen to the cancer cells, and thus exploits these normal physiological processes for its benefit. To do this, the cancer cells acquire the ability to orchestrate production of new vasculature by activating the 'angiogenic switch'. In doing so, they control non-cancerous cells that are present in the tumor that can form blood vessels by reducing the production of factors that inhibit blood vessel production, and increasing the production of factors that promote blood vessel formation.[7][12]
Tissue invasion and metastasis
- Cancer cells can break away from their site or organ of origin to invade surrounding tissue and spread (metastasize) to distant body parts.
One of the most well known properties of cancer cells is their ability to invade neighboring tissues. It is what dictates whether the tumor is benign or malignant, and is the reason for their dissemination around the body. The cancer cells have to undergo a multitude of changes in order for them to acquire the ability to metastasize. It is a multistep process that starts with local invasion of the cells into the surrounding tissues. They then have to invade blood vessels, survive in the harsh environment of the circulatory system, exit this system and then start dividing in the new tissue.[7][13]
Updates
In his 2010 NCRI conference talk, Hanahan proposed four new hallmarks. These were later codified in an updated review article entitled "Hallmarks of cancer: the next generation".[5]
- Deregulated metabolism
- Most cancer cells use abnormal metabolic pathways to generate energy, a fact appreciated since the early twentieth century with the postulation of the Warburg hypothesis,[14] but only now gaining renewed research interest.[15]
- Evading the immune system
- Cancer cells appear to be invisible to the body’s immune system.
- Unstable DNA
- Cancer cells generally have severe chromosomal abnormalities, which worsen as the disease progresses.
- Inflammation
- Recent discoveries have highlighted the role of local chronic inflammation in inducing many types of cancer.
Criticisms
An article in Nature Reviews Cancer in 2010 pointed out that five of the 'hallmarks' were also characteristic of benign tumours.[16] The only hallmark of malignant disease was its ability to invade and metastasize.[16]
An article in Journal of Biosciences in 2013 argued that original data for most of these hallmarks is lacking.[17] It argued that cancer is a tissue-level disease and these cellular-level hallmarks are misleading.
Notes and references
- ↑ Hanahan D, Weinberg RA (January 2000). "The Hallmarks of Cancer". Cell 100 (1): 57–70. doi:10.1016/S0092-8674(00)81683-9. PMID 10647931.
- 1 2 "Scientists Revisit 'Hallmarks of Cancer'". Science Daily. 16 March 2011. Retrieved 2011-04-04.
- ↑ "The Hallmarks of Cancer". Science Interviews. November 2010. Retrieved 2011-04-04.
- ↑ Hallmarks of Cancer, Cancer Research UK Science Update blog, November 2010
- 1 2 Hanahan, D.; Weinberg, R. A. (2011). "Hallmarks of Cancer: The Next Generation". Cell 144 (5): 646–674. doi:10.1016/j.cell.2011.02.013. PMID 21376230.
- ↑ Cell 100:59
- 1 2 3 4 5 6 Hanahan, D; Weinberg, RA (4 March 2011). "Hallmarks of cancer: the next generation.". Cell 144 (5): 646–74. doi:10.1016/j.cell.2011.02.013. PMID 21376230.
- ↑ Evan, GI; Vousden, KH (17 May 2001). "Proliferation, cell cycle and apoptosis in cancer.". Nature 411 (6835): 342–8. doi:10.1038/35077213. PMID 11357141.
- ↑ McClatchey, AI; Yap, AS (October 2012). "Contact inhibition (of proliferation) redux.". Current opinion in cell biology 24 (5): 685–94. doi:10.1016/j.ceb.2012.06.009. PMID 22835462.
- ↑ Elmore, S (June 2007). "Apoptosis: a review of programmed cell death.". Toxicologic pathology 35 (4): 495–516. doi:10.1080/01926230701320337. PMID 17562483.
- ↑ Greenberg, RA (March 2005). "Telomeres, crisis and cancer.". Current molecular medicine 5 (2): 213–8. doi:10.2174/1566524053586590. PMID 15974875.
- ↑ Bergers, G; Benjamin, LE (June 2003). "Tumorigenesis and the angiogenic switch.". Nature reviews. Cancer 3 (6): 401–10. doi:10.1038/nrc1093. PMID 12778130.
- ↑ van Zijl, F; Krupitza, G; Mikulits, W (2011). "Initial steps of metastasis: cell invasion and endothelial transmigration.". Mutation research 728 (1-2): 23–34. doi:10.1016/j.mrrev.2011.05.002. PMID 21605699.
- ↑ O. Warburg, K. Posener, E. Negelein: "Ueber den Stoffwechsel der Tumoren" Biochemische Zeitschrift, 152, pp. 319–344, 1924. (German). Reprinted in English in the book On metabolism of tumors by O. Warburg, Publisher: Constable, London, 1930.
- ↑ "Targeting tumour metabolism". Nature Reviews Drug Discovery 9 (7): 503–504. 2010. doi:10.1038/nrd3215. ISSN 1474-1776. PMID 20592733.
- 1 2 Lazebnik Y (April 2010). "What are the hallmarks of cancer?". Nat. Rev. Cancer 10 (4): 232–3. doi:10.1038/nrc2827. PMID 20355252.
- ↑ Sonnenschein C & Soto AM (Sep 2013). "The aging of the 2000 and 2011 Hallmarks of Cancer reviews: A critique" (PDF). J. Biosci 38 (3 date=Sept 2013): 651–63. PMID 23938395.
Bibliography
- Hanahan D, Weinberg RA (January 2000). "The hallmarks of cancer". Cell 100 (1): 57–70. doi:10.1016/S0092-8674(00)81683-9. PMID 10647931..
- Hanahan D, Weinberg RA (March 2011). "Hallmarks of cancer: the next generation". Cell 144 (5): 646–74. doi:10.1016/j.cell.2011.02.013. PMID 21376230..