Tetrafluoroammonium
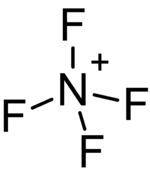
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula NF+
4. It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine.[1] Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane CF
4 and the tetrafluoroborate BF−
4 anion.
The tetrafluoroammonium ion forms salts with a large variety of fluorine-bearing anions. These include the bifluoride anion (HF−
2), tetrafluorobromate (BrF−
4), metal pentafluorides (XF−
5 where X is Ge, Sn, or Ti), hexafluorides (XF−
6 where X is P, As, Sb, Bi, or Pt), heptafluorides (XF−
7 where X is W, U, or Xe), octafluorides (XeF2−
8),[2] various oxyfluorides (XF
5O−
where X is W or U; FSO−
3, BrF
4O−
), and perchlorate (ClO−
4).[3] Attempts to make the nitrate salt, NF
4NO
3, were unsuccessful because of quick fluorination: NF+
4 + NO−
3 → NF
3 + FONO
2.[4]
Structure
The geometry of the tetrafluoroammonium ion is tetrahedral, with an estimated nitrogen-fluorine bond length of 124 pm. All fluorine atoms are in equivalent positions.[5]
Synthesis
Tetrafluoroammonium salts are prepared by oxidising nitrogen trifluoride with fluorine in the presence of a strong Lewis acid which acts as a fluoride ion acceptor. The original synthesis by Tolberg, Rewick, Stringham, and Hill in 1966 employs antimony pentafluoride as the Lewis acid:[5]
- NF
3 + F
2 + SbF
5 → NF
4SbF
6
The hexafluoroarsenate salt was also prepared by a similar reaction with arsenic pentafluoride at 120 °C:[5]
- NF
3 + F
2 + AsF
5 → NF
4AsF
6
The reaction of nitrogen trifluoride with fluorine and boron trifluoride at 800 °C yields the tetrafluoroborate salt:[6]
- NF
3 + F
2 + BF
3 → NF
4BF
4
NF+
4 salts can also be prepared by fluorination of NF
3 with krypton difluoride (KrF
2) and fluorides of the form MF
n, where M is Sb, Nb, Pt, Ti, or B. For example, reaction of NF
3 with KrF
2 and TiF
4 yields [NF+
4]
2TiF2−
6.[7]
Many tetrafluoroammonium salts can be prepared with metathesis reactions.
Reactions
Tetrafluoroammonium salts are extremely hygroscopic. The NF+
4 ion is readily hydrolysed into nitrogen trifluoride, H
2F+
, and oxygen gas:
- 2 NF+
4 + 2 H
2O → 2 NF
3 + 2 H
2F+
+ O
2
Some hydrogen peroxide (H
2O
2) is also formed during this process.[5]
Reaction of NF+
4SbF−
6 with alkali metal nitrates yields fluorine nitrate, FONO
2.[4]
Properties
Because tetrafluoroammonium salts are destroyed by water, it cannot be used as a solvent. Instead anhydrous hydrogen fluoride or bromine pentafluoride can be used as a to dissolve these salts.[8]
Tetrafluoroammonium salts usually have no colour. However some are coloured due to other metals in them. Red salts include (NF+
4)
2CrF2−
6, (NF+
4)
2NiF2−
6 and (NF+
4)
2PtF2−
6. (NF+
4)
2MnF2−
6, NF+
4UF−
7, NF+
4UOF−
5 and NF+
4XeF−
7 are yellow.[8]
Applications
NF+
4 salts are important for solid propellant NF
3–F
2 gas generators. They are also used as reagents for electrophilic fluorination of aromatic compounds in organic chemistry.[5]
See also
References
- ↑ Nikitin, I. V.; Rosolovskii, V. Y. (1985). "Tetrafluoroammonium Salts". Russian Chemical Reviews 54 (5): 426. Bibcode:1985RuCRv..54..426N. doi:10.1070/RC1985v054n05ABEH003068.
- ↑ Christe, K. O.; Wilson, W. W. (1982). "Perfluoroammonium and alkali-metal salts of the heptafluoroxenon(VI) and octafluoroxenon(VI) anions". Inorganic Chemistry 21 (12): 4113–4117. doi:10.1021/ic00142a001.
- ↑ Christe, K. O.; Wilson, W. W. (1986). "Synthesis and characterization of tetrafluoroammonium(1+) tetrafluorobromate(1-) and tetrafluoroammonium(1+) tetrafluorooxobromate(1-)". Inorganic Chemistry 25 (11): 1904–1906. doi:10.1021/ic00231a038.
- 1 2 Hoge, B.; Christe, K. O. (2001). "On the stability of NF+
4NO−
3 and a new synthesis of fluorine nitrate". Journal of Fluorine Chemistry 110 (2): 87–88. doi:10.1016/S0022-1139(01)00415-8. - 1 2 3 4 5 Sykes, A. G. (1989). Advances in Inorganic Chemistry. Academic Press. ISBN 0-12-023633-8.
- ↑ Patnaik, Pradyot (2002). Handbook of inorganic chemicals. McGraw-Hill Professional. ISBN 0-07-049439-8.
- ↑ John H. Holloway; Eric G. Hope (1998). A. G. Sykes, ed. Advances in Inorganic Chemistry. Academic Press. pp. 60–61. ISBN 0-12-023646-X.
- 1 2 Sykes, A. G. (1989-07-17). Advances in Inorganic Chemistry. Academic Press. p. 154. ISBN 9780080578828. Retrieved 22 June 2014.