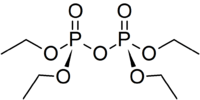
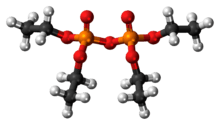
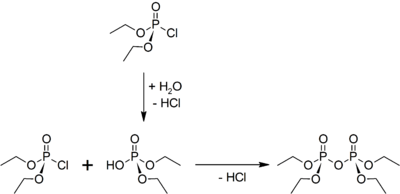Tetraethyl pyrophosphate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
tetraethyl diphosphate | |
| Identifiers | |
| 107-49-3 | |
| ChEMBL | ChEMBL293787 |
| ChemSpider | 7585 |
| Jmol interactive 3D | Image |
| PubChem | 7873 |
| |
| |
| Properties | |
| C8H20O7P2 | |
| Molar mass | 290.19 g·mol−1 |
| Appearance | colorless to amber liquid[1] |
| Odor | faint, fruity[1] |
| Density | 1.19 g/mL (20°C)[1] |
| Melting point | 0 °C; 32 °F; 273 K [1] |
| Boiling point | decomposes[1] |
| miscible[1] | |
| Vapor pressure | 0.0002 mmHg (20°C)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LDLo (Lowest published) |
0.5 mg/kg (rat, oral) 2.3 mg/kg (guinea pig, oral) 3 mg/kg (mouse, oral)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.05 mg/m3 [skin][1] |
| REL (Recommended) |
TWA 0.05 mg/m3 [skin][1] |
| IDLH (Immediate danger |
5 mg/m3[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetraethyl pyrophosphate, abbreviated TEPP, is an organophosphate compound. It is used as a pesticide.
This compound is a clear, colorless liquid. It is soluble in water, but hydrolyzes rapidly.[3] It was first synthesized by Philippe de Clermont. This compound may be prepared by two equivalents of diethyl chlorophosphate with one equivalent of water in the presence of pyridine to scavenge the hydrogen chloride formed:[4]
References
- 1 2 3 4 5 6 7 8 9 10 "NIOSH Pocket Guide to Chemical Hazards #0590". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "TEPP". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Robert L. Metcalf (2005), "Insect Control", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a14_263
- ↑ Toy, A. D. F. (1948). "The Preparation of Tetraethyl Pyrophosphate and Other Tetraalkyl Pyrophosphates". J. Am. Chem. Soc. 70 (11): 3882. doi:10.1021/ja01191a104.
This article is issued from Wikipedia - version of the Thursday, July 16, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.