Tetraazidomethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetraazidomethane | |||
| Identifiers | |||
| 869384-16-7 | |||
| ChemSpider | 17219283 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 16059578 | ||
| |||
| |||
| Properties | |||
| CN12 | |||
| Molar mass | 180.09 g/mol | ||
| Boiling point | ~165 °C (estimate) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Tetraazidomethane is a colorless, highly explosive liquid. Its chemical structure consists of a carbon atom substituted with four azide functional groups.
Synthesis
It was first prepared by Klaus Banert in 2006 by reaction of trichloroacetonitrile with sodium azide.[1]

Uses
As with other polyazides, tetraazidomethane has interest as a high-energy-density material with potential uses in explosives, propellants, or fireworks.[2] Silicon tetraazide is also a known compound.
Reactions
Banert has reported that tetraazidomethane participates in a number of surprising reactions including hydrolysis, cycloaddition reactions with alkenes and alkynes, and reaction with phosphines to form phosphazenes.[1]
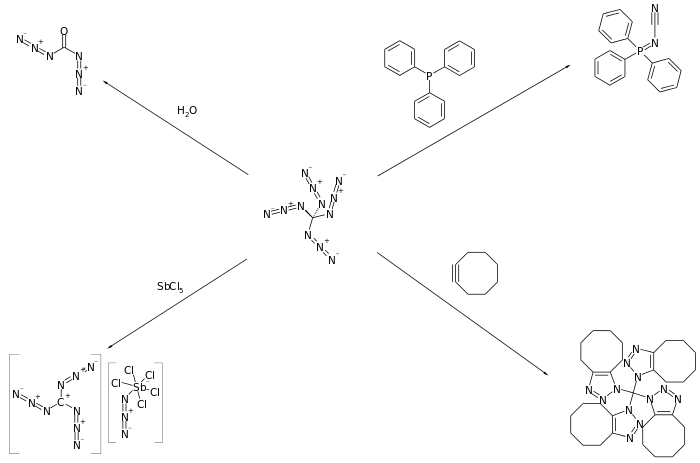
References
- 1 2 "The Exciting Chemistry of Tetraazidomethane", Klaus Banert, Young-Hyuk Joo, Tobias Ruffer, Bernhard Walfort, and Heinrich Lang, Angew. Chem. Int. Ed. 2007, 46, 1168–1171. doi:10.1002/anie.200603960
- ↑ "Tetraazidomethane: Chemistry with a Bang", Chemical & Engineering News, Dec. 18, 2006, 46.
This article is issued from Wikipedia - version of the Tuesday, January 21, 2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.

