External beam radiotherapy
| External beam radiotherapy | |
|---|---|
| Intervention | |
| ICD-10-PCS | ?0 |
| ICD-9-CM | 92.21-92.26 |


External beam radiotherapy (EBRT) or teletherapy is the most common form of radiotherapy. The patient sits or lies on a couch and an external source of radiation is pointed at a particular part of the body. In contrast to internal radiotherapy (brachytherapy), in which the radiation source is inside the body, external beam radiotherapy directs the radiation at the tumour from outside the body. Kilovoltage ("superficial") X-rays are used for treating skin cancer and superficial structures. Megavoltage ("deep") X-rays are used to treat deep-seated tumours (e.g. bladder, bowel, prostate, lung, or brain).
While X-ray and electron beams are by far the most widely used sources for external beam radiotherapy, a small number of centers operate experimental and pilot programs employing heavier particle beams, particularly proton sources.
Photons
Conventionally, the energy of diagnostic and therapeutic gamma- and X-rays is expressed in kilovolts or megavolts (kV or MV), whilst the energy of therapeutic electrons is expressed in terms of megaelectronvolts (MeV). In the first case, this voltage is the maximum electric potential used by a linear accelerator to produce the photon beam. The beam is made up of a spectrum of energies: the maximum energy is approximately equal to the beam's maximum electric potential times the electron charge. Thus a 1 MV beam will produce photons of no more than about 1 MeV. The mean X-ray energy is only about 1/3 of the maximum energy. Beam quality and hardness may be improved by special filters, which improve the homogeneity of the X-ray spectrum.
In the medical field, useful X-rays are produced when electrons are accelerated to a high energy. Some examples of X-ray energies used in medicine are:
- diagnostic X-rays - 20 to 150 keV
- superficial X-rays - 50 to 200 keV
- orthovoltage X-rays - 200 to 500 keV
- supervoltage X-rays - 500 to 1000 keV
- megavoltage X-rays - 1 to 25 MeV
Megavoltage X-rays are by far most common in radiotherapy for treatment of a wide range of cancers. Superficial and orthovoltage X-rays have application for the treatment of cancers at or close to the skin surface.[1]
Medically useful photon beams can also be derived from a radioactive source such as iridium-192, caesium-137 or radium-226 (which is no longer used clinically), or cobalt-60. Such photon beams, derived from radioactive decay, are more or less monochromatic and are properly termed gamma rays. The usual energy range is between 300 keV to 1.5 MeV, and is specific to the isotope.
Therapeutic radiation is mainly generated in the radiotherapy department using the following equipment:
- Orthovoltage units. These are also known as "deep" and "superficial" machines depending on their energy range. Orthovoltage units have essentially the same design as diagnostic X-ray machines. These machines are generally limited to less than 600 kV.
- Linear accelerators ("linacs") which produce megavoltage X-rays. The first use of a linac for medical radiotherapy was in 1953 (see also radiotherapy). Commercially available medical linacs produce X-rays and electrons with an energy range from 4 MeV up to around 25 MeV. The X-rays themselves are produced by the rapid deceleration of electrons in a target material, typically a tungsten alloy, which produces an X-ray spectrum via bremsstrahlung radiation. The shape and intensity of the beam produced by a linac may be modified or collimated by a variety of means. Thus, conventional, conformal, intensity-modulated, tomographic, and stereotactic radiotherapy are all produced by specially-modified linear accelerators.
- Cobalt units which produce stable, dichromatic beams of 1.17 and 1.33 MeV, resulting in an average beam energy of 1.25 MeV. The role of the cobalt unit has partly been replaced by the linear accelerator, which can generate higher energy radiation. Cobalt treatment still has a useful role to play in certain applications (for example the Gamma Knife) and is still in widespread use worldwide, since the machinery is relatively reliable and simple to maintain compared to the modern linear accelerator.
Electrons
X-rays are generated by bombarding a high atomic number material with electrons. If the target is removed (and the beam current decreased) a high energy electron beam is obtained. Electron beams are useful for treating superficial lesions because the maximum of dose deposition occurs near the surface. The dose then decreases rapidly with depth, sparing underlying tissue. Electron beams usually have nominal energies in the range 4-20 MeV. Depending on the energy this translates to a treatment range of approximately 1–5 cm (in water-equivalent tissue). Energies above 18 MeV are used very rarely. Although the X-ray target is removed in electron mode, the beam must be fanned out by sets of thin scattering foils in order to achieve flat and symmetric dose profiles in the treated tissue.
Hadron therapy
Hadron therapy involves the therapeutic use of protons, neutrons, and heavier ions (fully ionized atomic nuclei). Of these, proton therapy is by far the most common, though still quite rare compared to other forms of external beam radiotherapy.
Multi-leaf collimator
A typical multi-leaf collimator (MLC) consists of 2 sets of 40-80 leaves, each around 5mm to 10mm thick and several cm in the other two dimensions. Newer MLCs now have up to 160 leaves. Each leaf in the MLC is aligned parallel to the radiation field and can be moved independently to block part of the field. This allows the dosimetrist to match the radiation field to the shape of the tumor (by adjusting the position of the leaves), thus minimizing the amount of healthy tissue being exposed to radiation. On a machine without an MLC this must be accomplished using several hand-crafted blocks.
Intensity modulated radiation therapy
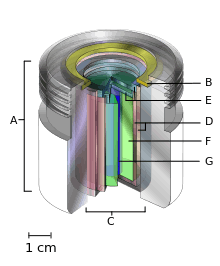
A.) an international standard source holder (usually lead),
B.) a retaining ring, and
C.) a teletherapy "source" composed of
D.) two nested stainless steel canisters welded to
E.) two stainless steel lids surrounding
F.) a protective internal shield (usually uranium metal or a tungsten alloy) and
G.) a cylinder of radioactive source material, often but not always cobalt-60. The diameter of the "source" is 30 mm.
Intensity modulated radiation therapy (IMRT) is an advanced radiotherapy technique used to minimize the amount of normal tissue being irradiated in the treatment field. In some systems this intensity modulation is achieved by moving the leaves in the MLC during the course of treatment, thereby delivering a radiation field with a non-uniform (i.e. modulated) intensity. With IMRT, radiation oncologists are able to break up the radiation beam into many "beamlets." This allows radiation oncologists to vary the intensity of each beamlet. With IMRT, doctors are often able to further limit the amount of radiation received by healthy tissue near the tumor. Doctors have found this sometimes allowed them to safely give a higher dose of radiation to the tumor, potentially increasing the chance of a cure.[2]
Image-guided radiation therapy
Image-guided radiation therapy (IGRT) augments radiotherapy with imaging to increase the accuracy and precision of target localization, thereby reducing the amount of healthy tissue in the treatment field. The more advanced the treatment techniques become in terms of dose deposition accuracy, the higher become the requirements for IGRT. In order to allow patients to benefit from sophisticated treatment techniques as IMRT or Hadron Therapy, patient alignment accuracies of 0.5 mm and less become desirable. Therefore, new methods like stereoscopic digital kilovoltage imaging based patient position verification (PPVS) [3] to alignment estimation based on in-situ Cone-Beam CT enrich the range of modern IGRT approaches.
See also
- CyberKnife
- Gamma knife
- Tomotherapy
- Radiation therapy
- IOERT
- IORT
- Brachytherapy
- Boron neutron capture therapy
References
- ↑ Advances in kilovoltage x-ray beam dosimetry in http://iopscience.iop.org/0031-9155/59/6/R183/article
- ↑ http://www.rtanswers.com/treatmentinformation/treatmenttypes/externalbeamradiation.aspx
- ↑ Boris Peter Selby, Georgios Sakas et al. (2007) 3D Alignment Correction for Proton Beam Treatment. In: Proceedings of Conf. of the German Society for Biomedical Engineering (DGBMT). Aachen.
General references
- Radiotherapy physics in practice, edited by JR Williams and DI Thwaites, Oxford University Press UK (2nd edition 2000), ISBN 0-19-262878-X
- Linear Particle Accelerator (Linac)Animation by Ionactive
- Superficial radiation therapy
- NATIONAL INSTITUTE OF RADIOLOGICAL SCIENCES (Japan)
| ||||||||||||||||||||||||||||||||||||||||||||||