Technetium (99mTc) albumin aggregated
| Identifiers | |
|---|---|
| ATC code | V09GA04 |
| ChemSpider | none |
| (verify) | |
Technetium 99mTc albumin aggregated (99mTc-MAA) is an injectable radiopharmaceutical used in nuclear medicine. It consists of a sterile aqueous suspension of Technetium-99m (99mTc) labeled to human albumin aggregate particles in the pH range of 3.8 to 8.0.
Preparation
Kits for preparing 99mTc-MAA are available in the United States from only a single manufacturer; Draximage. The kits are delivered to nuclear pharmacies as lyophilized powders of non-radioactive ingredients sealed under nitrogen. A nuclear pharmacist adds anywhere from 50 - 100 mCi of Na99mTc to the reaction vial to make the final product. After being allowed to set at room temperature for 15 minutes to ensure maximum tagging of 99mTc to the human albumin, the kit can then be diluted with sterile normal saline as needed.
Once prepared the product will have a turbid white appearance.
Quality control
Particle Size: No less than 90% of MAA particles can be between 10 - 90 micrometres in size and no particles may exceed 150 micrometres. Radiochemical purity: No less than 90% of the radioactivity present in the product must be tagged to albumin particles. Thus, no more than 10% soluble impurities may be present. Both of the above quality controls must be met before the product can be used in humans.
Indications, Dosage & Imaging
- Perfusion lung imaging to assess the presence of pulmonary emboli
- Isotope venography to identify lower extremity venous thrombosis
- Assessment of peritoneovenous (LeVeen) shunt patency
The typical adult dose for a lung imaging study is 3-4 mCi (containing between 100,000 - 250,000 albumin particles). The particle burden should be lowered for most pediatric patients and lowered to 50,000 for infants. The use of more than 250,000 particles in a dose is controversial as little extra data is acquired from such scans while there is certainly an increased risk of toxicity. Patients with pulmonary hypertension should be administered a minimum amount of particles to achieve a lung scan (i.e. 60,000). In any patient by administering a greater amount of particles than necessary for the diagnostic procedure increases the risks of toxicity.
Because of gravity effects, patients administered 99mTc MAA should be in the supine position to ensure as even a distribution of particles throughout the lungs as possible.
The total percentage of particles trapped in the lungs can be determined through a whole body scan after the administration of 99mTc MAA through the equation 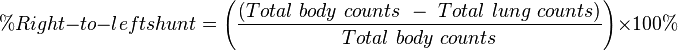 .
.
Expiration
Most 99mTc-MAA kits expire 6-8 hours after the Na99mTc has been added. See specific manufacturers inserts for specific expirations.
References
- Kowalsky, Richard J., Steven W. Falen. Radiopharmaceuticals in Nuclear Pharmacy and Nuclear Medicine. 2nd Edition.American Pharmacist Association: 2004.