Taxillusin
Taxillusin
 |
| Names |
| IUPAC name
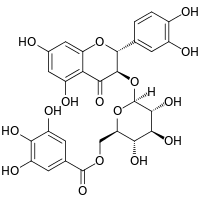
(2R,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-3,4-dihydro-2H-chromen-3-yl 6-O-(3,4,5-trihydroxybenzoyl)-β-D-glucopyranoside |
| Identifiers |
| ChemSpider |
10306084 |
| Jmol interactive 3D |
Image |
InChI=1S/C28H26O16/c29-11-6-14(32)19-17(7-11)42-25(9-1-2-12(30)13(31)3-9)26(22(19)37)44-28-24(39)23(38)21(36)18(43-28)8-41-27(40)10-4-15(33)20(35)16(34)5-10/h1-7,18,21,23-26,28-36,38-39H,8H2/t18-,21-,23+,24-,25-,26+,28+/m1/s1 Key: XUJNKPZDIVKHBE-GKOVUGPKSA-N InChI=1/C28H26O16/c29-11-6-14(32)19-17(7-11)42-25(9-1-2-12(30)13(31)3-9)26(22(19)37)44-28-24(39)23(38)21(36)18(43-28)8-41-27(40)10-4-15(33)20(35)16(34)5-10/h1-7,18,21,23-26,28-36,38-39H,8H2/t18-,21-,23+,24-,25-,26+,28+/m1/s1 Key: XUJNKPZDIVKHBE-GKOVUGPKBZ
|
Oc1cc(cc(O)c1O)C(=O)OC[C@H]5O[C@@H](O[C@H]2C(=O)c4c(O)cc(O)cc4O[C@@H]2c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]5O
|
| Properties |
| |
C28H26O16 |
| Molar mass |
618.50 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
|
| Infobox references |
|
|
Taxillusin is a flavonol found in the parasitic plant Taxillus kaempferi.[1][2] It is a galloylated 3-O-glucoside of quercetin.
References
- ↑ Konishi T, Nishio T, Kiyosawa S, Fujiwara Y and Konoshima T (February 1996). "The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of Taxillus kaempferi". Yakugaku Zasshi (in Japanese) 116 (2): 148–157.
- ↑ Atsushi Sakurai and Yasuaki Okumura (1983). "Chemical studies on the mistletoe. V. The structure of taxillusin, a new flavonoid glycoside isolated from Taxillus kaempferi". Bulletin of the Chemical Society of Japan 56 (2): 542–544. doi:10.1246/bcsj.56.542.
External links
Flavonols and their conjugates |
|---|
| | Backbone | |
|---|
| | Flavonols | Aglycones | |
|---|
| Conjugates | | |
|---|
| |
- Afzelin (Kaempferol 3-rhamnoside)
- Astragalin (kaempferol 3-O-glucoside)
- Kaempferitrin (kaempferol 3,7-dirhamnoside)
- Juglanin (Kaempferol 3-O-arabinoside)
- Kaempferol 3-alpha-L-arabinopyranoside
- Kaempferol 3-alpha-D-arabinopyranoside
- Kaempferol 7-alpha-L-arabinoside
- Kaempferol 7-O-glucoside
- Kaempferol 3-lathyroside
- Kaempferol 4'-rhamnoside
- Kaempferol 5-rhamnoside
- Kaempferol 7-rhamnoside
- Kaempferol 7-O-alpha-L-rhamnofuranoside
- Kaempferol 3-xyloside
- Kaempferol 7-xyloside
- Robinin (kaempferol-3-O-robinoside-7-O-rhamnoside)
- Kaempferol 3-O-rutinoside
- Sophoraflavonoloside (Kaempferol 3-O-sophoroside)
- Trifolin (Kaempferol 3-O-beta-D-galactoside)
|
|---|
| | |
|---|
| | |
|---|
|
|---|
|
|---|
| | O-Methylated flavonols | Aglycones | |
|---|
| Glycosides | of isorhamnetin |
- Narcissin (Isorhamnetin 3-O-rutinoside)
- Isorhamnetin 3-O-glucoside
- Tamarixetin 7-rutinoside
|
|---|
| other |
- Azalein (Azaleatin 3-O-α-L-rhamnoside)
- Centaurein (Centaureidin 7-O-glucoside)
- Eupalin (Eupalitin 3-0-rhamnoside)
- Eupatolin (Eupatolitin 3-O-rhamnoside)
- Jacein (Jaceidin 7-O-glucoside)
- Patulitrin (Patuletin 7-O-glucoside
- Xanthorhamnin (Rhamnetin glycoside)
|
|---|
|
|---|
|
|---|
| | Derivative flavonols | Aglycones |
- Noricaritin
- Dihydronoricaritin
|
|---|
| Glycosides | |
|---|
|
|---|
| | Pyranoflavonols | |
|---|
| | Furanoflavonols | |
|---|
| | Semisynthetic | |
|---|
|
