Tantalum pentoxide
 | |
| Names | |
|---|---|
| IUPAC name
Tantalum(V) oxide | |
| Systematic IUPAC name
Ditantalum pentaoxide | |
| Identifiers | |
| 1314-61-0 | |
| ChemSpider | 452513 |
| Jmol interactive 3D | Image |
| PubChem | 518712 |
| |
| |
| Properties | |
| Ta2O5 | |
| Molar mass | 441.893 g/mol |
| Appearance | white, odorless powder |
| Density | β-Ta2O5 = 8.18 g/cm3[1] α-Ta2O5 = 8.37 g/cm3 |
| Melting point | 1,872 °C (3,402 °F; 2,145 K) |
| negligible | |
| Solubility | insoluble in organic solvents and most mineral acids, reacts with HF |
| Band gap | 3.8 - 5.3 eV |
| Refractive index (nD) |
2.275 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tantalum pentoxide, also known as tantalum(V) oxide, is the inorganic compound with the formula Ta
2O
5. It is a white solid that is insoluble in all solvents but is attacked by strong bases and hydrofluoric acid. Ta
2O
5 is an inert material with a high refractive index and low absorption (i.e. colourless), which makes it useful for coatings.[2] It is also extensively used in the production of capacitors, due to its high dielectric constant.
Preparation
Occurrence and Refining
Tantalum occurs in the minerals tantalite and columbite (columbium being an archaic name for niobium), which occur in pegmatites, an igneous rock formation. Mixtures of columbite and tantalite are called coltan. Tantalite was discovered by Anders Gustaf Ekeberg at Ytterby, Sweden, and Kimoto, Finland. The minerals microlite and pyrochlore contain approximately 70% and 10% Ta, respectively.
Tantalum ores often contain significant amounts of niobium, which is itself a valuable metal. As such, both metals are extracted so that they may be sold. The overall process is one of hydrometallurgy and begins with a leaching step; in which the ore is treated with hydrofluoric acid and sulfuric acid to produce water-soluble hydrogen fluorides, such as the heptafluorotantalate. This allows the metals to be separated from the various non-metallic impurities in the rock.
The tantalum and niobium hydrogenflorides are then removed from the aqueous solution by liquid-liquid extraction using organic solvents, such as cyclohexanone or methyl isobutyl ketone. This step allows the simple removal of various metal impurities (e.g. iron and manganese) which remain in the aqueous phase in the form of fluorides. Separation of the tantalum and niobium is then achieved by pH adjustment. Niobium requires a higher level of acidity to remain soluble in the organic phase and can hence be selectively removed by extraction into less acidic water. The pure tantalum hydrogen fluoride solution is then neutralised with aqueous ammonia to give hydrated tantalum oxide (Ta2O5(H2O)x), which is calcinated to tantalum pentoxide (Ta2O5) as described in these idealized equations:[3]
From alkoxides
Tantalum oxide is frequently used in electronics, often in the form of thin films. For these applications it can be produced by MOCVD (or related techniques), which involves the hydrolysis of its volatile halides or alkoxides:
- Ta2(OEt)10 + 5 H2O → Ta2O5 + 10 EtOH
- 2 TaCl5 + 5 H2O → Ta2O5 + 10 HCl
Structure and properties
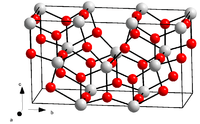
The crystal structure of tantalum pentoxide has been the matter of some debate. The bulk material is disordered,[4] being either amorphous or polycrystalline; with single crystals being difficult to grow. As such Xray crystallography has largely been limited to powder diffraction, which provides less structural information.
At least 2 polymorphs are known to exist. A low temperature form, known as L- or β-Ta2O5, and the high temperature form known as H- or α-Ta2O5. The transition between these two forms is slow and reversible; taking place between 1000-1360 °C, with a mixture of structures existing at intermediate temperatures.[4] The structures of both polymorphs consist of chains built from octahedral TaO6 and pentagonal bipyramidal TaO7 polyhedra sharing opposite vertices; which are further joined by edge-sharing.[5][6] The overall crystal system is orthorhombic in both cases, with the space group of β-Ta2O5 being identified as Pna2 by single crystal X-ray diffraction.[7] A high pressure form (Z-Ta2O5) has also been reported, in which the Ta atoms adopt a 7 coordinate geometry to give a monoclinic structure (space group C2).[8]
The difficulty in forming material with a uniform structure has led to variations in its reported properties. Like many metal oxides Ta2O5 is an insulator and its band gap has variously been reported as being between 3.8 to 5.3 eV, depending on the method of manufacture.[9][10][11] In general the more amorphous the material the greater its observed band gap. It should be noted that these observed values are significantly higher than those predicted by computational chemistry (2.3 - 3.8 eV).[12][13][14]
Its dielectric constant is typically about ~25[15] although values of over 50 have been reported.[16] In general tantalum pentoxide is considered to be a high-k dielectric material.
Reactions
Ta2O5 does not react appreciably with either HCl or HBr, however it will dissolve in hydrofluoric acid, and reacts with potassium bifluoride and HF according to the following equation:[17][18]
- Ta2O5 + 4 KHF2 + 6 HF → 2 K4[TaF7] + 5 H2O
Ta2O5 can be reduced to metallic Ta via the use of metallic reductants such as calcium and aluminium.
- Ta2O5 + 5 Ca → 2 Ta + 5 CaO

Uses
In electronics
Owing to its high band gap and dielectric constant, tantalum pentoxide has found a variety of uses in electronics, particularly in tantalum capacitors. These are used in automotive electronics, cell phones, and pagers, electronic circuitry; thin-film components; and high-speed tools. In the 1990s, interest grew in the use of tantalum oxide as a high-k dielectric for DRAM capacitor applications.,[19][20]
It is used in on-chip metal-insulator-metal capacitors for high frequency CMOS integrated circuits. Tantalum oxide may have applications as the charge trapping layer for Non-volatile memories.,[21][22] There are applications of tantalum oxide in resistive switching memories.[23]
Other uses
Due to its high refractive index, Ta2O5 has been utilized in the fabrication of the glass of photographic lenses.[2][24]
References
- ↑ Reisman, Arnold; Holtzberg, Frederic; Berkenblit, Melvin; Berry, Margaret (20 September 1956). "Reactions of the Group VB Pentoxides with Alkali Oxides and Carbonates. III. Thermal and X-Ray Phase Diagrams of the System K2O or K2CO3 with Ta2O5". Journal of the American Chemical Society 78 (18): 4514–4520. doi:10.1021/ja01599a003.
- 1 2 Fairbrother, Frederick (1967). The Chemistry of Niobium and Tantalum. New York: Elsevier Publishing Company. pp. 1–28. ISBN 978-0-444-40205-9.
- ↑ Anthony Agulyanski (2004). "Fluorine chemistry in the processing of tantalum and niobium". In Anatoly Agulyanski. Chemistry of Tantalum and Niobium Fluoride Compounds (1st ed.). Burlington: Elsevier. ISBN 9780080529028.
- 1 2 Askeljung, Charlotta; Marinder, Bengt-Olov; Sundberg, Margareta (1 November 2003). "Effect of heat treatment on the structure of L-Ta2O5:". Journal of Solid State Chemistry 176 (1): 250–258. doi:10.1016/j.jssc.2003.07.003.
- ↑ Stephenson, N. C.; Roth, R. S. (1971). "Structural systematics in the binary system Ta2O5–WO3. V. The structure of the low-temperature form of tantalum oxide L-Ta2O5". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 27 (5): 1037–1044. doi:10.1107/S056774087100342X.
- ↑ Wells, A.F. (1947). Structural Inorganic Chemistry. Oxford: Clarendon Press.
- ↑ Wolten, G. M.; Chase, A. B. (1 August 1969). "Single-crystal data for β Ta2O5 and A KPO3". Zeitschrift für Kristallographie 129 (5-6): 365–368. doi:10.1524/zkri.1969.129.5-6.365.
- ↑ Zibrov, I. P.; Filonenko, V. P.; Sundberg, M.; Werner, P.-E. (1 August 2000). "Structures and phase transitions of B-Ta2O5 and Z-Ta2O5: two high-pressure forms of Ta2O5". Acta Crystallographica Section B Structural Science 56 (4): 659–665. doi:10.1107/S0108768100005462.
- ↑ Kukli, Kaupo; Aarik, Jaan; Aidla, Aleks; Kohan, Oksana; Uustare, Teet; Sammelselg, Väino (1995). "Properties of tantalum oxide thin films grown by atomic layer deposition". Thin Solid Films 260 (2): 135–142. doi:10.1016/0040-6090(94)06388-5.
- ↑ Fleming, R. M.; Lang, D. V.; Jones, C. D. W.; Steigerwald, M. L.; Murphy, D. W.; Alers, G. B.; Wong, Y.-H.; van Dover, R. B.; Kwo, J. R.; Sergent, A. M. (1 January 2000). "Defect dominated charge transport in amorphous Ta2O5 thin films". Journal of Applied Physics 88 (2): 850. doi:10.1063/1.373747. Cite uses deprecated parameter
|coauthors=(help) - ↑ Murawala, Prakash A.; Sawai, Mikio; Tatsuta, Toshiaki; Tsuji, Osamu; Fujita, Shizuo; Fujita, Shigeo (1993). "Structural and Electrical Properties of Ta2O5 Grown by the Plasma-Enhanced Liquid Source CVD Using Penta Ethoxy Tantalum Source". Japanese Journal of Applied Physics 32 (Part 1, No. 1B): 368–375. doi:10.1143/JJAP.32.368.
- ↑ Ramprasad, R. (1 January 2003). "First principles study of oxygen vacancy defects in tantalum pentoxide". Journal of Applied Physics 94 (9): 5609. doi:10.1063/1.1615700.
- ↑ Sawada, H.; Kawakami, K. (1 January 1999). "Electronic structure of oxygen vacancy in Ta2O5]". Journal of Applied Physics 86 (2): 956. doi:10.1063/1.370831.
- ↑ Nashed, Ramy; Hassan, Walid M. I.; Ismail, Yehea; Allam, Nageh K. (2013). "Unravelling the interplay of crystal structure and electronic band structure of tantalum oxide (Ta2O5)". Physical Chemistry Chemical Physics. doi:10.1039/C2CP43492J.
- ↑ Macagno, V.; Schultze, J.W. (1 December 1984). "The growth and properties of thin oxide layers on tantalum electrodes". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 180 (1-2): 157–170. doi:10.1016/0368-1874(84)83577-7.
- ↑ Hiratani, M.; Kimura, S.; Hamada, T.; Iijima, S.; Nakanishi, N. (1 January 2002). "Hexagonal polymorph of tantalum–pentoxide with enhanced dielectric constant". Applied Physics Letters 81 (13): 2433. doi:10.1063/1.1509861.
- ↑ Agulyansky, A. "Potassium fluorotantalate in solid, dissolved and molten conditions" J. Fluorine Chemistry 2003, 155-161. doi:10.1016/S0022-1139(03)00190-8
- ↑ Brauer, Georg (1965). Handbook of preparative inorganic chemistry. [S.l.]: Academic Press. p. 256. ISBN 978-0-12-395591-3.
- ↑ Ezhilvalavan, S.; Tseng, T. Y. (1999). "Preparation and properties of tantalum pentoxide (Ta2O5) thin films for ultra large scale integrated circuits (ULSIs) application - a review". Journal of Materials Science: Materials in Electronics 10 (1): 9–31. doi:10.1023/A:1008970922635.
- ↑ Chaneliere, C; Autran, J L; Devine, R A B; Balland, B (1998). "Tantalum pentoxide (Ta2O5) thin films for advanced dielectric applications". Materials Science and Engineering: R 22 (6): 269–322. doi:10.1016/S0927-796X(97)00023-5.
- ↑ Wang, X; et al. (2004). "A Novel MONOS-Type Nonvolatile Memory Using High-
 Dielectrics for Improved Data Retention and Programming Speed". IEEE Transactions on Electron Devices 51 (4): 597. doi:10.1109/TED.2004.824684.
Dielectrics for Improved Data Retention and Programming Speed". IEEE Transactions on Electron Devices 51 (4): 597. doi:10.1109/TED.2004.824684. - ↑ Zhu, H; et al. (2013). "Design and Fabrication of Ta2O5 Stacks for Discrete Multibit Memory Application". IEEE Transactions on Nanotechnology 12 (6): 1151–1157. doi:10.1109/TNANO.2013.2281817.
- ↑ Lee, M-.J; et al. (2011). "A fast, high-endurance and scalable non-volatile memory device made from asymmetric Ta2O5-x/TaO2-x bilayer structures". Nature Materials 10: 625. doi:10.1038/NMAT3070.
- ↑ Musikant, Solomon (1985). "Optical Materials: An Introduction to Selection and Application". CRC Press: 28. ISBN 978-0-8247-7309-0.
|chapter=ignored (help)
| ||||||