Tannin


A tannin (also known as vegetable tannin, natural organic tannins, or sometimes tannoid, i.e. a type of biomolecule, as opposed to modern synthetic tannin) is an astringent, plant polyphenolic compound that binds to and precipitates proteins and various other organic compounds including amino acids and alkaloids.
The term tannin (from tanna, an Old High German word for oak or fir tree, as in Tannenbaum) refers to the use of wood tannins from oak in tanning animal hides into leather; hence the words "tan" and "tanning" for the treatment of leather. However, the term "tannin" by extension is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with various macromolecules.
The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation, and perhaps also as pesticides, and in plant growth regulation.[1] The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit or red wine.[2] Likewise, the destruction or modification of tannins with time plays an important role in the ripening of fruit and the aging of wine.
Tannins have molecular weights ranging from 500 to over 3,000[3] (gallic acid esters) and up to 20,000 (proanthocyanidins).
Structure and classes of tannins
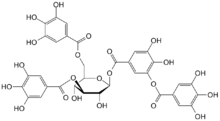
There are three major classes of tannins:[4] Shown below are the base unit or monomer of the tannin. Particularly in the flavone-derived tannins, the base shown must be (additionally) heavily hydroxylated and polymerized in order to give the high molecular weight polyphenol motif that characterizes tannins. Typically, tannin molecules require at least 12 hydroxyl groups and at least five phenyl groups to function as protein binders.
| Base Unit: |  |
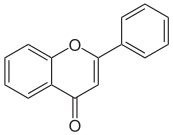 |
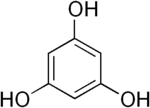 |
|---|---|---|---|
| Class/Polymer: | Hydrolyzable tannins | Non-Hydrolyzable or condensed tannins |
Phlorotannins |
| Sources | Plants | Plants | Brown algae |
Oligostilbenoids (oligo- or polystilbenes) are oligomeric forms of stilbenoids and constitute a class of tannins.[5]
Pseudo tannins
Pseudo tannins are low molecular weight compounds associated with other compounds. They do not change color during the Goldbeater's skin test, unlike hydrolysable and condensed tannins, and cannot be used as tanning compounds.[4] Some examples of pseudo tannins and their sources are:[6]
| Pseudo tannin | Source(s) |
|---|---|
| Gallic acid | Rhubarb |
| Flavan-3-ols (Catechins) | Tea, acacia, catechu, cocoa, guarana |
| Chlorogenic acid | Nux-vomica, coffee, mate |
| Ipecacuanhic acid | Carapichea ipecacuanha |
History
Ellagic acid, gallic acid, and pyrogallic acid were first discovered by chemist Henri Braconnot in 1831.[7]:20 Julius Löwe was the first person to synthesize ellagic acid by heating gallic acid with arsenic acid or silver oxide.[7]:20 [8]
Maximilian Nierenstein studied natural phenols and tannins[9] found in different plant species. Working with Arthur George Perkin, he prepared ellagic acid from algarobilla and certain other fruits in 1905.[10] He suggested its formation from galloyl-glycine by Penicillium in 1915.[11] Tannase is an enzyme that Nierenstein used to produce m-digallic acid from gallotannins.[12] He proved the presence of catechin in cocoa beans in 1931.[13] He showed in 1945 that luteic acid, a molecule present in the myrobalanitannin, a tannin found in the fruit of Terminalia chebula, is an intermediary compound in the synthesis of ellagic acid.[14]
At these times, molecule formulas were determined through combustion analysis. The discovery in 1943 by Martin and Synge of paper chromatography provided for the first time the means of surveying the phenolic constituents of plants and for their separation and identification. There was an explosion of activity in this field after 1945, none more so than that of Edgar Charles Bate-Smith and Tony Swain[15] at Cambridge University.
In 1966, Edwin Haslam proposed a first comprehensive definition of plant polyphenols based on the earlier proposals of Bate-Smith, Swain and Theodore White, which includes specific structural characteristics common to all phenolics having a tanning property. It is referred to as the White–Bate-Smith–Swain–Haslam (WBSSH) definition.[16]
Occurrence
Tannins are distributed in species throughout the plant kingdom. They are commonly found in both gymnosperms as well as angiosperms. Mole[17] (1993) studied the distribution of tannin in 180 families of dicotyledons and 44 families of monocotyledons (Cronquist). Most families of dicot contain tannin-free species (tested by their ability to precipitate proteins).
The best known families of which all species tested contain tannin are: Aceraceae, Actinidiaceae, Anacardiaceae, Bixaceae, Burseraceae, Combretaceae, Dipterocarpaceae, Ericaceae, Grossulariaceae, Myricaceae for dicot and Najadaceae and Typhaceae in Monocot. To the family of the oak, Fagaceae, 73% of the species tested (N = 22) contain tannin. For those of acacias, Mimosaceae, only 39% of the species tested (N = 28) contain tannin, among Solanaceae rate drops to 6% and 4% for the Asteraceae. Some families like the Boraginaceae, Cucurbitaceae, Papaveraceae contain no tannin-rich species.
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants, and comprising up to 50% of the dry weight of leaves. Tannins of tropical woods tend to be of a cathetic nature rather than of the gallic type present in temperate woods.[18]
There may be a loss in the bio-availability of still other tannins in plants due to birds, pests, and other pathogens.[19]
Localization in plant organs
Tannins are found in leaf, bud, seed, root, and stem tissues. An example of the location of the tannins in stem tissue is that they are often found in the growth areas of trees, such as the secondary phloem and xylem and the layer between the cortex and epidermis. Tannins may help regulate the growth of these tissues.
Cellular localization
In all vascular plants studied so far, tannins are manufactured by a chloroplast-derived organelle, the tannosome.[20] Tannins are mainly physically located in the vacuoles or surface wax of plants. These storage sites keep tannins active against plant predators, but also keep some tannins from affecting plant metabolism while the plant tissue is alive; it is only after cell breakdown and death that the tannins are active in metabolic effects.
Tannins are classified as ergastic substances, i.e., non-protoplasm materials found in cells. Tannins, by definition, precipitate proteins. In this condition, they must be stored in organelles able to withstand the protein precipitation process. Idioblasts are isolated plant cells which differ from neighboring tissues and contain non-living substances. They have various functions such as storage of reserves, excretory materials, pigments, and minerals. They could contain oil, latex, gum, resin or pigments etc. They also can contain tannins. In Japanese persimmon (Diospyros kaki) fruits, tannin is accumulated in the vacuole of tannin cells, which are idioblasts of parenchyma cells in the flesh.[21]
Presence in soils
The convergent evolution of tannin-rich plant communities has occurred on nutrient-poor acidic soils throughout the world. Tannins were once believed to function as anti-herbivore defenses, but more and more ecologists now recognize them as important controllers of decomposition and nitrogen cycling processes. As concern grows about global warming, there is great interest to better understand the role of polyphenols as regulators of carbon cycling, in particular in northern boreal forests.
Leaf litter and other decaying parts of a kauri (Agathis australis), a tree species found in New Zealand, decompose much more slowly than those of most other species. Besides its acidity, the plant also bears substances such as waxes and phenols, most notably tannins,[22] that are harmful to microorganisms.
Presence in water and wood

The leaching of highly water soluble tannins from decaying vegetation and leaves along a stream may produce what is known as a blackwater river. Water flowing out of bogs has a characteristic brown color from dissolved peat tannins. The presence of tannins (or humic acid) in well water can make it smell bad or taste bitter, but this does not make it unsafe to drink.[23]

Tannins leaching from an unprepared driftwood decoration in an aquarium can cause pH lowering and coloring of the water to a tea-like tinge. A way to avoid this is to boil the wood in water several times, discarding the water each time. Using peat as an aquarium substrate can have the same effect.
Many hours of boiling the driftwood may need to be followed by many weeks or months of constant soaking and many water changes before the water will stay clear. Adding baking soda to the water to raise its pH level will accelerate the process of leaching, as the more alkaline solution can draw out tannic acid from the wood faster than the pH-neutral water.[24]
Softwoods, while in general much lower in tannins than hardwoods,[25] are usually not recommended for use in an aquarium[26] so using a hardwood with a very light color, indicating a low tannin content, can be an easy way to avoid tannins. Tannic acid is brown in color, so in general white woods have a low tannin content. Woods with a lot of yellow, red, or brown coloration to them (like southern yellow pine, cedar, redwood, red oak, etc.) tend to contain a lot of tannin.[27]
Extraction
There is no single protocol for extracting tannins from all plant material. The procedures used for tannins are widely variable.[28] It may be that acetone in the extraction solvent increases the total yield by inhibiting interactions between tannins and proteins during extraction[28] or even by breaking hydrogen bonds between tannin-protein complexes.[29]
Tests for tannins
There are three groups of methods for the analysis of tannins: precipitation of proteins or alkaloids, reaction with phenolic rings, and depolymerization.[30]
- Goldbeater's skin test
When goldbeater's skin or ox skin is dipped in HCl, rinsed in water, soaked in the tannin solution for 5 minutes, washed in water, and then treated with 1% FeSO4 solution, it gives a blue black color if tannin was present.
It is rather a test for phenolics in general. Powdered plant leaves of the test plant (1.0 g) are weighed into a beaker and 10 ml of distilled water are added. The mixture is boiled for five minutes. Two drops of 5% FeCl3 are then added. Production of a greenish precipitate was an indication of the presence of tannins.[31] Alternatively, a portion of the water extract is diluted with distilled water in a ratio of 1:4 and few drops of 10% ferric chloride solution is added. A blue or green color indicates the presence of tannins (Evans, 1989).[32]
- Other methods
The hide-powder method is used in tannin analysis for leather tannin and the Stiasny method for wood adhesives.[33][34] Statistical analysis reveals that there is no significant relationship between the results from the hide-powder and the Stiasny methods.[35][36]
- hide-powder method
400 mg of sample tannins are dissolved in 100 ml of distilled water. 3 g of slightly chromated hide-powder previously dried in vacuum for 24h over CaCl2 are added and the mixture stirred for 1 h at ambient temperature. The suspension is filtered without vacuum through a sintered glass filter. The weight gain of the hide-powder expressed as a percentage of the weight of the starting material is equated to the percentage of tannin in the sample.
- Stiasny's method
100 mg of sample tannins are dissolved in 10 ml distilled water. 1 ml of 10M HCl and 2 ml of 37% formaldehyde are added and the mixture heated under reflux for 30 min. The reaction mixture is filtered while hot through a sintered glass filter. The precipitate is washed with hot water (5x 10 ml) and dried over CaCl2. The yield of tannin is expressed as a percentage of the weight of the starting material.
Reaction with phenolic rings
The bark tannins of Commiphora angolensis have been revealed by the usual color and precipitation reactions and by quantitative determination by the methods of Löwenthal-Procter and of Deijs[37] (formalin-hydrochloric acid method).[38]
Colorimetric methods have existed such as the Neubauer-Löwenthal method which uses potassium permanganate as an oxidizing agent and indigo sulfate as an indicator, originally proposed by Löwenthal in 1877.[39] The difficulty is that the establishing of a titer for tannin is not always convenient since it is extremely difficult to obtain the pure tannin. Neubauer proposed to remove this difficulty by establishing the titer not with regard to the tannin but with regard to crystallised oxalic acid, whereby he found that 83 g oxalic acid correspond to 41.20 g tannin. Löwenthal's method has been criticized. For instance, the amount of indigo used is not sufficient to retard noticeably the oxidation of the non-tannins substances. The results obtained by this method are therefore only comparative.[40][41] A modified method, proposed in 1903 for the quantification of tannins in wine, Feldmann's method, is making use of calcium hypochlorite, instead of potassium permanganate, and indigo sulfate.[42][43]
Food items with tannins
Pomegranates
Berries

Most berries, such as cranberries,[44] strawberries and blueberries,[45] contain both hydrolyzable and condensed tannins.
Nuts
Nuts that can be consumed raw, such as hazelnuts, walnuts, and pecans, contain high amounts of tannins. Almonds have a lower content. Tannin concentration in the crude extract of these nuts did not directly translate to the same relationships for the condensed fraction.[46]
Herbs and spices
Cloves, tarragon, cumin, thyme, vanilla, and cinnamon all contain tannins.
Legumes
Most legumes contain tannins. Red-colored beans contain the most tannins, and white-colored beans have the least. Peanuts without shells have a very low tannin content. Chickpeas (garbanzo beans) have a smaller amount of tannins.[47]
Chocolate
Chocolate liquor contains about 6% tannins.[48]
Drinks with tannins
Principal human dietary sources of tannins are tea and coffee.[49] Most wines aged in charred oak barrels possess tannins absorbed from the wood.[50] This concentration gives wine its signature bitterness.[51]
Coffee pulp has been found to contain low to trace amounts of tannins.[52]
Fruit juices
Although citrus fruits do not themselves contain tannins, orange-colored juices often contain food dyes with tannins. Apple juice, grape juices and berry juices are all high in tannins. Sometimes tannins are even added to juices and ciders to create a more astringent feel to the taste.
Beer
In addition to the alpha acids extracted from hops to provide bitterness in beer, condensed tannins are also present. These originate both from the malt and hops. Especially in Germany, trained brewmasters consider the presence of tannins as a flaw. In some styles, the presence of this astringency is acceptable or even desired, as, for example, in a Flanders red ale.
In lager type beers, the tannins can form a precipitate with specific haze-forming proteins in the beer resulting in turbidity at low temperature. This chill haze can be prevented by removing part of the tannins or part of the haze-forming proteins. Tannins are removed using PVPP, haze-forming proteins by using silica or tannic acid.[53]
Health effects of tannins
Properties for animal nutrition
Tannins have traditionally been considered antinutritional, but it is now known that their beneficial or antinutritional properties depend upon their chemical structure and dosage. The new technologies used to analyze molecular and chemical structures have shown that a division into condensed and hydrolyzable tannins is too simplistic.[54] Recent studies have demonstrated that products containing chestnut tannins included at low dosages (0.15–0.2%) in the diet of chickens may be beneficial.[55]
Some studies suggest that chestnut tannins have positive effects on silage quality in the round bale silages, in particular reducing NPNs (non protein nitrogen) in the lowest wilting level.[56]
Improved fermentability of soya meal nitrogen in the rumen may occur.[57] Studies by S. Gonzalez et al. (2002)[58] on in vitro ammonia release and dry matter degradation of soybean meal comparing three different types of tannins (quebracho, acacia and chestnut) demonstrated that chestnut tannins are more efficient in protecting soybean meal from in vitro degradation by rumen bacteria.
Condensed tannins inhibit herbivore digestion by binding to consumed plant proteins and making them more difficult for animals to digest, and by interfering with protein absorption and digestive enzymes (for more on that topic, see plant defense against herbivory). Many tannin-consuming animals secrete a tannin-binding protein (mucin) in their saliva. Tannin-binding capacity of salivary mucin is directly related to its proline content. Advantages in using salivary proline-rich proteins (PRPs) to inactivate tannins are:
- PRPs inactivate tannins to a greater extent than do dietary proteins; this results in reduced fecal nitrogen losses
- PRPs contain non specific nitrogen and nonessential amino acids; this makes them more convenient for an animal to exploit rather than using up valuable dietary protein
Histatins, another type of salivary proteins, also precipitate tannins from solution, thus preventing alimentary adsorption.[59]
Tannin market

Tannin production began at the beginning of the 19th century with the industrial revolution, to produce tanning material for the need for more leather. Before that time, processes used plant material and were long (up to six months).
There was a collapse in the vegetable tannin market in the 1950s–1960s, due to the appearance of synthetic tannins, which were invented in response to a scarcity of vegetable tannins during World War II. At that time, many small tannin industry sites closed.[60] Vegetable tannins are estimated to be used for the production of 10–20% of the global leather production.
The cost of the final product depends on the method used to extract the tannins, in particular the use of solvents, alkali and other chemicals used (for instance glycerin). For large quantities, the most cost-effective method is hot water extraction.
Tannic acid is used worldwide as clarifying agent in alcoholic drinks and as aroma ingredient in both alcoholic and soft drinks or juices. Tannins from different botanical origins also find extensive uses in the wine industry.
Uses
Tannins are an important ingredient in the process of tanning leather. Tanbark from oak, mimosa, chestnut and quebracho tree has traditionally been the primary source of tannery tannin, though inorganic tanning agents are also in use today and account for 90% of the world's leather production.[61]
Tannins produce different colors with ferric chloride (either blue, blue black, or green to greenish-black) according to the type of tannin. Iron gall ink is produced by treating a solution of tannins with iron(II) sulfate.
Tannin is a component in a type of industrial particleboard adhesive developed jointly by the Tanzania Industrial Research and Development Organization and Forintek Labs Canada.[62] Pinus radiata tannins has been investigated for the production of wood adhesives.[63]
Condensed tannins, e.g., quebracho tannin, and Hydrolyzable tannins, e.g., chestnut tannin, appear to be able to substitute a high proportion of synthetic phenol in phenol-formaldehyde resins for wood particleboard.
Tannins can be used for production of anti-corrosive primer, sold under brand name-Nox Primer for treatment of rusted steel surfaces prior to painting, rust converter to transform oxidized steel into a smooth sealed surface and rust inhibitor.
The use of resins made of tannins has been investigated to remove mercury and methylmercury from solution.[64] Immobilized tannins have been tested to recover uranium from seawater.[65]
See also
References
- ↑ Katie E. Ferrell; Thorington, Richard W. (2006). Squirrels: the animal answer guide. Baltimore: Johns Hopkins University Press. p. 91. ISBN 0-8018-8402-0.
- ↑ McGee, Harold (2004). On food and cooking: the science and lore of the kitchen. New York: Scribner. p. 714. ISBN 0-684-80001-2.
- ↑ Bate-Smith and Swain (1962). "Flavonoid compounds". In Florkin M., Mason H. S. Comparative biochemistry III. New York: Academic Press. pp. 75–809.
- 1 2 Notes on Tannins from PharmaXChange.info
- ↑ Boralle, N; Gottlieb, H.E; Gottlieb, O.R; Kubitzki, K; Lopes, L.M.X; Yoshida, M; Young, M.C.M (1993). "Oligostilbenoids from Gnetum venosum". Phytochemistry 34 (5): 1403–1407. doi:10.1016/0031-9422(91)80038-3.
- ↑ Ashutosh Kar (2003). Pharmacognosy And Pharmacobiotechnology. New Age International. pp. 44–. ISBN 978-81-224-1501-8. Retrieved 31 January 2011.
- 1 2 Grasser, Georg (1922). Synthetic Tannins. F. G. A. Enna. (trans.). ISBN 9781406773019.
- ↑ Löwe, Zeitschrift für Chemie, 1868, 4, 603
- ↑ Drabble, E.; Nierenstein, M. (1907). "On the Rôle of Phenols, Tannic Acids, and Oxybenzoic Acids in Cork Formation". Biochemical Journal 2 (3): 96–102.1. PMC 1276196. PMID 16742048.
- ↑ Perkin, A. G.; Nierenstein, M. (1905). "CXLI.—Some oxidation products of the hydroxybenzoic acids and the constitution of ellagic acid. Part I". Journal of the Chemical Society, Transactions 87: 1412. doi:10.1039/CT9058701412.
- ↑ Nierenstein, M. (1915). "The Formation of Ellagic Acid from Galloyl-Glycine by Penicillium". The Biochemical Journal 9 (2): 240–244. doi:10.1042/bj0090240. PMC 1258574. PMID 16742368.
- ↑ Nierenstein, M. (1932). "A biological synthesis of m-digallic acid". The Biochemical Journal 26 (4): 1093–1094. doi:10.1042/bj0261093. PMC 1261008. PMID 16744910.
- ↑ Adam, W. B.; Hardy, F.; Nierenstein, M. (1931). "The Catechin of the Cacao Bean". Journal of the American Chemical Society 53 (2): 727–728. doi:10.1021/ja01353a041.
- ↑ Nierenstein, M.; Potter, J. (1945). "The distribution of myrobalanitannin". The Biochemical Journal 39 (5): 390–392. doi:10.1042/bj0390390. PMC 1258254. PMID 16747927.
- ↑ Haslam, Edwin (2007). "Vegetable tannins – Lessons of a phytochemical lifetime". Phytochemistry 68 (22–24): 2713–2721. doi:10.1016/j.phytochem.2007.09.009. PMID 18037145.
- ↑ Quideau, Stéphane (22 September 2009). "Why bother with Polyphenols". Groupe Polyphenols. Retrieved 21 August 2012.
- ↑ Simon Mole (1993). "The Systematic Distribution of Tannins in the Leaves of Angiosperms: A Tool for Ecological Studies". Biochemical Systematics and Ecology 21 (8): 833–846. doi:10.1016/0305-1978(93)90096-A.
- ↑ Tannin in Tropical Woods. Doat J, Bois. For Tmp., 1978, volume 182, pages 34-37
- ↑ Kadam, S. S.; Salunkhe, D. K.; Chavan, J. K. (1990). Dietary tannins: consequences and remedies. Boca Raton: CRC Press. p. 177. ISBN 0-8493-6811-1.
- ↑ "The tannosome is an organelle forming condensed tannins in the chlorophyllous organs of Tracheophyta". Jean-Marc Brillouet, Charles Romieu, Benoît Schoefs, Katalin Solymosi, Véronique Cheynier, Hélène Fulcrand, Jean-Luc Verdeil and Geneviève Conéjéro, Annals of Botany, September 11, 2013, doi:10.1093/aob/mct168
- ↑ Identification of Molecular Markers Linked to the Trait of Natural Astringency Loss of Japanese Persimmon (Diospyros kaki) Fruit. Shinya Kanzaki, Keizo Yonemori and Akira Sugiura, J. Amer. Soc. Hort. Sci., 2001, 126(1), pages 51–55 (article)
- ↑ Eric Verkaik, Anne G. Jongkindet, Frank Berendse "Short-term and long-term effects of tannins on nitrogen mineralisation and litter decomposition in kauri (Agathis australis (D. Don) Lindl.) forests". Plant And Soil 2006; Volume 287, Numbers 1–2, pages 337–345 doi:10.1007/s11104-006-9081-8
- ↑ Tannins, lignins and humic acids in well water on www.gov.ns.ca
- ↑ Preparing Driftwood for Your Freshwater Aquarium
- ↑ Polyflavonoid tannins — a main cause of soft-rot failure in CCA-treated timber
- ↑ Driftwood Do's & Don'ts
- ↑ Tannin and hardwood flooring
- 1 2 The Tannin Handbook, Ann E. Hagerman, 1988 (book)
- ↑ "Condensed tannins". Porter L. J., 1989, in Natural Products of Woody Plants I, Rowe J. W. (ed), Springer-Verlag: Berlin, Germany, pages 651–690
- ↑ Quantitative Methods for the Estimation of Tannins in Plant Tissues. Augustin Scalbert, Plant Polyphenols, Basic Life Sciences, 1992, Volume 59, pages 259-280, doi:10.1007/978-1-4615-3476-1_15
- ↑ "Antibacterial activity of leave extracts of Nymphaea lotus (Nymphaeaceae) on Methicillin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Staphylococcus aureus (VRSA) isolated from clinical samples". Akinjogunla O. J., Yah C. S., Eghafona N. O. and Ogbemudia F. O., Annals of Biological Research, 2010, 1 (2), pages 174–184
- ↑ "Phytochemical Analysis and Antimicrobial Activity Of Scoparia dulcis and Nymphaea lotus". Jonathan Yisa, Australian Journal of Basic and Applied Sciences, 2009, 3(4): pages 3975–3979
- ↑ Tannin analysis of Acacia mearnsii bark - a comparison of the hide-powder and Stiasny methods. Zheng G.C., Lin Y.L. and Yazaki Y., ACIAR Proceedings Series, 1991, No. 35, pages 128-131 (abstract)
- ↑ Study on Fast Determination Content of Condensed Tannin Using Stiasny Method. Chen Xiangming,Chen Heru and Li Weibin, Guangdong Chemical Industry, 2006-07 (abstract)
- ↑ Guangcheng, Zheng; Yunlu, Lin; Yazaki, Y. (1991). "Bark tannin contents of Acacia mearnsii provenances and the relationship between the hide-powder and the Stiasny methods of estimation". Australian Forestry 54 (4): 209–211. doi:10.1080/00049158.1991.10674579.
- ↑ Leather Chemists' Pocket-Book: A Short Compendium of Analytical Methods. Henry Richardson Procter, Edmund Stiasny and Harold Brumwel, E. & F.N. Spon, Limited, 1912 - 223 pages (book at google books)
- ↑ Chemical study of bark from Commiphora angolensis Engl. Cardoso Do Vale, J., Bol Escola Farm Univ Coimbra Edicao Cient, 1962, volume 3, page 128 (abstract)
- ↑ Catechins isolated from tea leaves. W. B. Deijs, Recueil des Travaux Chimiques des Pays-Bas, 1939, Volume 58, Issue 9, pages 805–830, doi:10.1002/recl.19390580907
- ↑ Uber die Bestimmung des Gerbstoffs. J. Lowenthal, Z. Anal. Chem, 1877, volume 16, pages 33-48, doi:10.1007/BF01355993
- ↑ The Estimation of Tannin in Cider. C. W. Spiers, The Journal of Agricultural Science, January 1914, Volume 6, Issue 01, pages 77-83, doi:10.1017/S0021859600002173
- ↑ Notes on Löwenthal's method for the determination of tannin. Harry Snyder, J. Am. Chem. Soc., 1893, volume 15, issue 10, pages 560–563, doi:10.1021/ja02120a004
- ↑ Nouvelle methode de dosage du tannin. Schweizerische Wochenschrift für Chemie und Pharmacie, page 329 (article in French)
- ↑ Journ. de Pharma. et de Chimie, 1903, page 528
- ↑ Vattem D. A., Ghaedian R., Shetty K. (2005). "Enhancing health benefits of berries through phenolic antioxidant enrichment: focus on cranberry" (PDF). Asia Pac J Clin Nutr 14 (2): 120–30. PMID 15927928.
- ↑ Puupponen-Pimiä R., Nohynek L., Meier C.; et al. (April 2001). "Antimicrobial properties of phenolic compounds from berries". J. Appl. Microbiol. 90 (4): 494–507. doi:10.1046/j.1365-2672.2001.01271.x. PMID 11309059.
- ↑ http://google.com/search?q=cache:K-qF4vdf8a8J:www.icomst.helsinki.fi/icomst2008/Paper%2520CD/General%2520speakers%2Bposters-3p%2520papers/Session2/2B/2B.3.Amarowicz.pdf+tannin+%22nuts%22&hl=en&ct=clnk&cd=10&gl=us
- ↑ Reed J. D. (1 May 1995). "Nutritional toxicology of tannins and related polyphenols in forage legumes". J. Anim. Sci. 73 (5): 1516–28. PMID 7665384.
- ↑ Robert L. Wolke; Marlene Parrish (29 March 2005). What Einstein told his cook 2: the sequel: further adventures in kitchen science. W. W. Norton & Company. p. 433. ISBN 978-0-393-05869-7.
- ↑ Clifford MN (2004). "Diet-derived phenols in plasma and tissues and their implications for health". Planta Med 70 (12): 1103–14. doi:10.1055/s-2004-835835. PMID 15643541.
- ↑ Tao Y, García JF, Sun DW (2014). "Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process". Crit Rev Food Sci Nutr 54 (6): 817–35. doi:10.1080/10408398.2011.609949. PMID 24345051.
- ↑ McRae JM, Kennedy JA (2011). "Wine and grape tannin interactions with salivary proteins and their impact on astringency: a review of current research". Molecules 16 (3): 2348–64. doi:10.3390/molecules16032348. PMID 21399572.
- ↑ Clifford M. N., Ramirez-Martinez J. R. (1991). "Tannins in wet-processed coffee beans and coffee pulp". Food Chemistry 40 (2): 191–200. doi:10.1016/0308-8146(91)90102-T.
- ↑ http://www.natural-specialities.com/natural-specialities/PDF/Applications/BR02%20overview%20fact%20sheet%20version%202.1.pdf
- ↑ Muller-Harvey I., McAllan A. B. (1992). "Tannins: Their biochemistry and nutritional properties". Adv. Plant Cell Biochem. And Biotechnol. 1: 151–217.
- ↑ Schiavone A., Guo K., Tassone S.; et al. (March 2008). "Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks". Poult. Sci. 87 (3): 521–7. doi:10.3382/ps.2007-00113. PMID 18281579.
- ↑ Tabacco E., Borreani G., Crovetto G. M., Galassi G., Colombo D., Cavallarin L. (1 December 2006). "Effect of chestnut tannin on fermentation quality, proteolysis, and protein rumen degradability of alfalfa silage". J. Dairy Sci. 89 (12): 4736–46. doi:10.3168/jds.S0022-0302(06)72523-1. PMID 17106105.
- ↑ Mathieu F., Jouany J. P. (1993). "Effect of chestnut tannin on the fermentability of soyabean meal nitrogen in the rumen". Ann Zootech 42 (2): 127. doi:10.1051/animres:19930210.
- ↑ González S., Pabón M. L., Carulla J. (2002). "Effects of tannins on in vitro ammonia release and dry matter degradation of soybean meal". Arch. Latinoam. Prod. Anim. 10 (2): 97–101.
- ↑ Salivary proteins as a defense against dietary tannins. Shimada T. Journal of Chemical Ecology 2006 Jun;32(6):1149-63.
- ↑ "The Status of Mangrove Ecosystems: Trends in the Utilisation and Management of Mangrove Resources". D. Macintosh and S. Zisman
- ↑ Marion Kite; Roy Thomson (2006). Conservation of leather and related materials. Butterworth-Heinemann. p. 23. ISBN 978-0-7506-4881-3.
- ↑ Bisanda E. T. N., Ogola W. O., Tesha J. V. (August 2003). "Characterisation of tannin resin blends for particle board applications". Cement and Concrete Composites 25 (6): 593–8. doi:10.1016/S0958-9465(02)00072-0.
- ↑ Li, Jingge; Maplesden, Frances (1998). "Commercial production of tannins from radiata pine bark for wood adhesives" (PDF). IPENZ Transactions 25 (1/EMCh).
- ↑ Torres J., Olivares S., De La Rosa D., Lima L., Martínez F., Munita C. S., Favaro D. I. T. (1999). "Removal of mercury(II) and methylmercury from solution by tannin adsorbents". Journal of Radioanalytical and Nuclear Chemistry 240 (1): 361–5. doi:10.1007/BF02349180.
- ↑ Takashi Sakaguchia, Akira Nakajimaa (June 1987). "Recovery of Uranium from Seawater by Immobilized Tannin". Separation Science and Technology 22 (6): 1609–23. doi:10.1080/01496398708058421.
External links
| Wikisource has the text of The New Student's Reference Work article Tannin. |
- Tannins: fascinating but sometimes dangerous molecules
- Tannin Chemistry PDF (1.41 MB)
- Haslam, Edwin (1989). Plant polyphenols: vegetable tannins revisited. CUP Archive. ISBN 978-0-521-32189-1.
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
|
