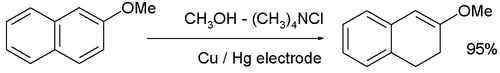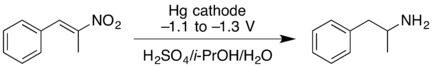Electrosynthesis
Electrosynthesis in chemistry is the synthesis of chemical compounds in an electrochemical cell.[1][2] The main advantage of electrosynthesis over an ordinary redox reaction is avoidance of the potential wasteful other half-reaction and the ability to precisely tune the required potential. Electrosynthesis is actively studied as a science and also has many industrial applications.
Experimental setup
The basic setup in electrosynthesis is a galvanic cell, a potentiostat and two electrodes. Good electrosynthetic conditions use a solvent and electrolyte combination that minimizes electrical resistance.[3] Protic conditions often use alcohol-water or dioxane-water solvent mixtures with an electrolyte such as a soluble salt, acid or base. Aprotic conditions often use an organic solvent such as acetonitrile or dichloromethane with electrolytes such as lithium perchlorate or tetrabutylammonium acetate. Electrodes are selected which provide favorable electron transfer properties towards the substrate while maximizing the activation energy for side reactions. This activation energy is often related to an overpotential of a competing reaction. For example, in aqueous conditions the competing reactions in the cell are the formation of oxygen at the anode and hydrogen at the cathode. In this case a graphite anode and lead cathode could be used effectively because of their high overpotentials for oxygen and hydrogen formation respectively. Many other materials can be used as electrodes. Other examples include platinum, magnesium, mercury (as a liquid pool in the reactor), stainless steel or reticulated vitreous carbon. Some reactions use a sacrificial electrode is used which is consumed during the reaction like zinc or lead. The two basic cell types are undivided cell or divided cell type. In divided cells the cathode and anode chambers are separated with a semiporous membrane. Common membrane materials include sintered glass, porous porcelain, polytetrafluoroethene or polypropylene. The purpose of the divided cell is to permit the diffusion of ions while restricting the flow of the products and reactants. This is important when unwanted side reactions are possible. An example of a reaction requiring a divided cell is the reduction of nitrobenzene to phenylhydroxylamine, where the latter chemical is susceptible to oxidation at the anode.
Reactions
Organic oxidations take place at the anode with initial formation of radical cations as reactive intermediates. Compounds are reduced at the cathode to radical anions. The initial reaction takes place at the surface of the electrode and then the intermediates diffuse into the solution where they participate in secondary reactions.
The yield of an electrosynthesis is expressed both in terms the chemical yield and current efficiency. Current efficiency is the ratio of Coulombs consumed in forming the products to the total number of Coulombs passed through the cell. Side reactions decrease the current efficiency.
The potential drop between the electrodes determines the rate constant of the reaction. Electrosynthesis is carried out with either constant potential or constant current. The reason one chooses one over the other is due to a trade off of ease of experimental conditions versus current efficiency. Constant potential uses current more efficiently because the current in the cell decreases with time due to the depletion of the substrate around the working electrode (stirring is usually necessary to decrease the diffusion layer around the electrode). This is not the case under constant current conditions however. Instead as the substrate's concentration decreases the potential across the cell increases in order to maintain the fixed reaction rate. This consumes current in side reactions produced outside the target voltage.
Anodic oxidations
- The most well-known electrosynthesis is the Kolbe electrolysis, in which two carboxylic acids decarboxylate, and the remaining structures bond together:
- A variation is called the non-Kolbe reaction when a heteroatom (nitrogen or oxygen) is present at the α-position. The intermediate oxonium ion is trapped by a nucleophile usually solvent.
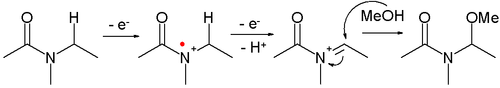
- Amides can be oxidized to N-acyliminium ions, which can be captured by various nucleophiles, for example:
- This reaction type is called a Shono oxidation. An example is the α-methoxylation of N-carbomethoxypyrrolidine[4]
- Oxidation of a carbanion can lead to a coupling reaction for instance in the electrosynthesis of the tetramethyl ester of ethanetetracarboxylic acid from the corresponding malonate ester[5]
- α-amino acids form nitriles and carbon dioxide via oxidative decarboxylation at AgO anodes (the latter is formed in-situ by oxidation of Ag2O):[3][6]
- Cyanoacetic acid from cathodic reduction of carbon dioxide and anodic oxidation of acetonitrile.[7]
Cathodic reductions
- In the Markó–Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by electroreducing their toluate ester.
- The cathodic hydroisomerization of activated olefins is applied industrially in the synthesis of adiponitrile from 2 equivalents of acrylonitrile:
- The cathodic reduction of arene compounds to the 1,4-dihydro derivatives is similar to a Birch reduction. Examples from industry are the reduction of phthalic acid:
and the reduction of 2-methoxynaphthalene:
- The Tafel rearrangement, named for Julius Tafel, was at one time an important method for the synthesis of certain hydrocarbons from alkylated ethyl acetoacetate, a reaction accompanied by the rearrangement reaction of the alkyl group:[8][9]
- Cathodic reduction of a nitroalkene can give the oxime in good yield. At higher negative reduction potentials, the nitroalkene can be reduced further, giving the primary amine but with lower yield.[11]
- An electrochemical carboxylation of a para-isobutylbenzyl chloride to Ibuprofen is promoted under supercritical carbon dioxide.[12]
- Cathodic reduction of a carboxylic acid (oxalic acid) to an aldehyde (glyoxylic acid, shows as the rare aldehyde form) in a divided cell:[13][14]
- An electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic acid;[15] this conversion uses carbon dioxide as a feedstock to generate oxalic acid.
Electrofluorination
In organofluorine chemistry, many perfluorinated compounds are prepared by electrochemical synthesis, which is conducted in liquid HF at voltages near 5–6 V using Ni anodes. The method was invented in the 1930s.[16] Amines, alcohols, carboxylic acids, and sulfonic acids are converted to the perfluorinated derivatives using this technology. A solution or suspension of the hydrocarbon in hydrogen fluoride is electrolyzed at 5–6 V to produce high yields of the perfluorinated product.
External links
- Electrochemistry Encyclopedia Link
References
- ↑ The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules Jeffrey B. Sperry and Dennis L. Wright Chem. Soc. Rev., 2006, 35, 605 – 621, doi:10.1039/b512308a
- ↑ Topics in current chemistry. Electrochemistry, Vol. 3 (Topics in Current Chemistry, Vol. 148) E. Steckhan (Ed), Springer, NY 1988.
- 1 2 Grimshaw, James (2000). Electrochemical Reactions and Mechanisms in Organic Chemistry. Amsterdam: Elsevier Science. pp. 1–7, 282, & 310. ISBN 9780444720078.
- ↑ Organic Syntheses, Coll. Vol. 7, p.307 (1990); Vol. 63, p.206 (1985). Link
- ↑ Organic Syntheses, Coll. Vol. 7, p.482 (1990); Vol. 60, p.78 (1981) Link
- ↑ Hampson, N; Lee, J; MacDonald, K (1972). "The oxidation of amino compounds at anodic silver". Electrochimica Acta 17 (5): 921–955. doi:10.1016/0013-4686(72)90014-X.
- ↑ Barba, Fructuoso; Batanero, Belen (2004). "Paired Electrosynthesis of Cyanoacetic Acid". The Journal of Organic Chemistry 69 (7): 2423–2426. doi:10.1021/jo0358473.
- ↑ "Electrochemistry Encyclopedia – Tafel: his life and science". Archived from the original on 2012-02-06.
- ↑ Tafel, Julius; Hahl, Hans (1907). "Vollständige Reduktion des Benzylacetessigesters". Berichte der deutschen chemischen Gesellschaft 40 (3): 3312–3318. doi:10.1002/cber.190704003102.
- ↑ Krishnan, V.; Muthukumaran, A.; Udupa, H. V. K. (1979). "The electroreduction of benzyl cyanide on iron and cobalt cathodes". Journal of Applied Electrochemistry 9 (5): 657–659. doi:10.1007/BF00610957.
- ↑ Wessling, M.; Schäfer, H.J. (1991). "Cathodic reduction of 1-nitroalkenes to oximes and primary amines". Chem. Ber. 124: 2303–2306. doi:10.1002/cber.19911241024.
- ↑ Sakakura, Toshiyasu; Choi, Jun-Chul; Yasuda, Hiroyuki (13 June 2007). "Transformation of Carbon dioxide". Chemical Reviews (American Chemical Society) 107 (6): 2365–2387. doi:10.1021/cr068357u. PMID 17564481.
- ↑ Tafel, Julius; Friedrichs, Gustav (1904). "Elektrolytische Reduction von Carbonsäuren und Carbonsäureestern in schwefelsaurer Lösung". Berichte der deutschen chemischen Gesellschaft 37 (3): 3187–3191. doi:10.1002/cber.190403703116.
- ↑ Cohen, Julius (1910). Practical Organic Chemistry (PDF) (2nd ed.). London: Macmillan and Co. Limited. pp. 102–104.
- ↑ Bouwman, Elisabeth; Angamuthu, Raja; Byers, Philip; Lutz, Martin; Spek, Anthony L. (July 15, 2010). "Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex". Science 327 (5393): 313–315. doi:10.1126/science.1177981.
- ↑ J. H. Simons "Production of Fluorocarbons I. The Generalized Procedure and its Use with Nitrogen Compounds" Journal of The Electrochemical Society, 1949, Volume 95, pp. 47–52. doi: 10.1149/1.2776733 . See also related articles by Simons et al. on pages 53, 55, 59, and 64 of the same issue.
|


_Oxide_Amino_Acid_Oxidation.png)