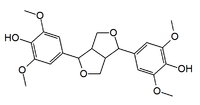
Syringaresinol
 | |
| Names | |
|---|---|
| IUPAC name
4,4'-(1S,3aR,4S,6aR)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diylbis(2,6-dimethoxyphenol) | |
| Other names
(+)-Syringaresinol (−)-Syringaresinol | |
| Identifiers | |
| 21453-69-0 | |
| ChemSpider | 391324 |
| Jmol interactive 3D | Image |
| PubChem | 443023 |
| |
| |
| Properties | |
| C22H26O8 | |
| Molar mass | 418.43 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Syringaresinol is a lignan found in Castela emoryi,[1] in Prunus mume[2] or in Magnolia thailandica.[3]
This compound inhibits Helicobacter pylori motility.[2]
Eleutheroside E, one of the glycosides isolated from the prickly eleutherococcus, is identical with acanthoside D, which is one of the glycosides isolated from the cluster-flowering acanthopanax and represents the di-β-D-glucoside of (−)-syringaresinol.[4]
Gallery
-
-syringaresinol.svg.png)
(+)-syringaresinol -O-β-D-glucoside (eletheroside E) A constituent of Eleutherococcus senticosus.
References
- ↑ Stöcklin, W.; De Silva, L.B.; Geissman, T.A. (1969). "Constituents of holacantha emoryi". Phytochemistry 8 (8): 1565. doi:10.1016/S0031-9422(00)85931-2.
- 1 2 Miyazawa, M; Utsunomiya, H; Inada, K; Yamada, T; Okuno, Y; Tanaka, H; Tatematsu, M (2006). "Inhibition of Helicobacter pylori motility by (+)-Syringaresinol from unripe Japanese apricot". Biological & Pharmaceutical Bulletin 29 (1): 172–3. doi:10.1248/bpb.29.172. PMID 16394533.
- ↑ Monthong (2011). "(+)-Syringaresinol Lignan from New Species Magnolia Thailandica". American Journal of Applied Sciences 8 (12): 1268. doi:10.3844/ajassp.2011.1268.1271.
- ↑ Identity of eleutheroside E with acanthoside D. Yu. S. Ovodov, G. M. Frolova, L. A. Elyakova and G. B. Elyakov, Bulletin of the Academy of Sciences of the USSR, Division of chemical science, November 1965, Volume 14, Issue 11, pages 2035-2036, doi:10.1007/BF00845912
| ||||||||||||||||||||||
This article is issued from Wikipedia - version of the Tuesday, July 07, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.