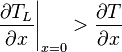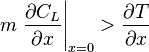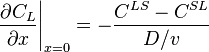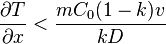Supercooling
Supercooling, also known as undercooling,[1] is the process of lowering the temperature of a liquid or a gas below its freezing point without it becoming a solid.
Explanation
A liquid crossing its standard freezing point will crystalize in the presence of a seed crystal or nucleus around which a crystal structure can form creating a solid. Lacking any such nuclei, the liquid phase can be maintained all the way down to the temperature at which crystal homogeneous nucleation occurs. Homogeneous nucleation can occur above the glass transition temperature, but if homogeneous nucleation has not occurred above that temperature an amorphous (non-crystalline) solid will form.
Water normally freezes at 273.15 K (0 °C or 32 °F) but it can be "supercooled" at standard pressure down to its crystal homogeneous nucleation at almost 224.8 K (−48.3 °C/−55 °F).[2][3] The process of supercooling requires that water be pure and free of nucleation sites, which can be achieved by processes like reverse osmosis, but the cooling itself does not require any specialised technique. If water is cooled at a rate on the order of 106 K/s, the crystal nucleation can be avoided and water becomes a glass. Its glass transition temperature is much colder and harder to determine, but studies estimate it at about 136 K (−137 °C/-215 °F).[4] Glassy water can be heated up to approximately 150 K (−123 °C/−189.4 °F) without nucleation occurring.[3] In the range of temperatures between 231 K (−42 °C/−43.6 °F) and 150 K (−123 °C/−189.4 °F) experiments find only crystal ice.
Droplets of supercooled water often exist in stratiform and cumulus clouds. Aircraft flying through these clouds see an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes, unless the aircraft are equipped with an appropriate de-icing system. Freezing rain is also caused by supercooled droplets.
The process opposite to supercooling, the melting of a solid above the freezing point, is much more difficult, and a solid will almost always melt at the same temperature for a given pressure. For this reason, it is the melting point which is usually identified, using melting point apparatus; even when the subject of a paper is "freezing-point determination", the actual methodology is "the principle of observing the disappearance rather than the formation of ice".[5] It is possible, at a given pressure, to superheat a liquid above its boiling point without it becoming gaseous.
Supercooling is often confused with freezing-point depression. Supercooling is the cooling of a liquid below its freezing point without it becoming solid. Freezing point depression is when a solution can be cooled below the freezing point of the corresponding pure liquid due to the presence of the solute; an example of this is the freezing point depression that occurs when sodium chloride is added to pure water.
Constitutional supercooling

Constitutional supercooling, which occurs during solidification, is due to compositional changes, and results in cooling a liquid below the freezing point ahead of the solid–liquid interface. When solidifying a liquid, the interface is often unstable, and the velocity of the solid–liquid interface must be small in order to avoid constitutional supercooling.
Supercooled zones are observed when the liquidus temperature gradient at the interface is larger than the temperature gradient.
or
The slope of the liquidus phase boundary on the phase diagram is 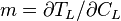
The concentration gradient is related to points, 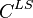 and
and 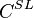 , on the phase diagram:
, on the phase diagram:
For steady-state growth 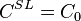 and the partition function
and the partition function  can be assumed to be constant. Therefore the minimum thermal gradient necessary to create a stable solid front is as expressed below.
can be assumed to be constant. Therefore the minimum thermal gradient necessary to create a stable solid front is as expressed below.
For more information, see the equation (3) of [6]
In animals
In order to survive extreme low temperatures in certain environments, some animals undergo forms of supercooling that allow them to remain unfrozen and avoid cell damage and death. There are many techniques that aid in supercooling, such as the production of antifreeze proteins, or AFPs, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice.[7] The winter flounder is one such fish that utilizes these proteins to survive in its frigid environment. Noncolligative proteins are secreted by the liver into the bloodstream.[8] Other animals use colligative antifreezes, which increases the concentration of solutes in their bodily fluids, thus lowering their freezing point. Fish that rely on supercooling for survival must also live well below the water surface, because if they came into contact with ice nuclei they would freeze immediately. Animals that undergo supercooling to survive must also remove ice-nucleating agents from their bodies because they act as a starting point for freezing. Supercooling is also common in insects, reptiles, and other ectotherms, with insects being able to survive in the coldest environments out of any supercooling animals.
It should be noted that supercooling is a last resort for animals. The best option is to move to a warmer environment if possible. As an animal gets farther and farther below its original freezing point the chance of spontaneous freezing increases dramatically for its internal fluids, as this is a thermodynamically unstable state. The fluids eventually reach the supercooling point, which is the temperature at which the supercooled solution freezes spontaneously due to being so far below its normal freezing point.[9] Animals unintentionally undergo supercooling and are only able to decrease the odds of freezing once supercooled. Even though supercooling is essential for survival, there are many risks associated with it.
Applications
One commercial application of supercooling is in refrigeration. Freezers can cool drinks to a supercooled level[10] so that when they are opened, they form a slush. Another example is a product that can supercool the beverage in a conventional freezer.[11] The Coca-Cola Company briefly marketed special vending machines containing Sprite in the UK, and Coke in Singapore, which stored the bottles in a supercooled state so that their content would turn to slush upon opening.[12]
Supercooling was successfully applied to organ preservation by the group of Martin Yarmush and Korkut Uygun in the Center for Engineering in Medicine at Massachusetts General Hospital/Harvard Medical School. Livers that were later transplanted into recipient animals were preserved by supercooling for up to 96 hours (4 days), quadrupling the limits of what could be achieved by conventional liver preservation methods. The livers were supercooled to a temperature of –6 °C in a specialized solution that protected against freezing and injury from the cold temperature.[13]
Another potential application is drug delivery. In 2015 researchers demonstrated the ability to crystallize membranes at a specific time. Liquid-encapsulated drugs can potentially be delivered to the site and with a slight environmental change, the liquid rapidly changes into a crystalline form that releases the drug.[14]
See also
References
- ↑ Rathz, Tom. "Undercooling". NASA. Archived from the original on 2010-01-12. Retrieved 2010-01-12.
- ↑ Moore, Emily; Valeria Molinero (24 November 2011). "structural transformation in supercooled water controls the crystallization rate of ice". Nature 479: 506–508. arXiv:1107.1622. Bibcode:2011Natur.479..506M. doi:10.1038/nature10586. Retrieved 24 November 2011.
- 1 2 Debenedetti & Stanley 2003, p. 42
- ↑ Insights into Phases of Liquid Water from Study of Its Unusual Glass-Forming Properties, C. Austen Angell, Science 319, 582 (2008); .
- ↑ "A new method of freezing-point determination for small quantities", J. A. Ramsay, J. Exp. Biol..1949; 26
- ↑ page from 99~100 Archived July 29, 2013 at the Wayback Machine
- ↑ J.G. Duman (2001). "Antifreeze and ice nucleator proteins in terrestrial arthropods". Annual Review of Physiology 63: 327–357. doi:10.1146/annurev.physiol.63.1.327. PMID 11181959.
- ↑ Garth L Fletcher, Choy L Hew, and Peter L Davies (2001). "Antifreeze Proteins of Teleost Fishes". Annual Review of Physiology 63: 359–390. doi:10.1146/annurev.physiol.63.1.359. PMID 11181960.
- ↑ C.H. Lowe, P.J. Lardner, and E.A. Halpern (1971). "Supercooling in reptiles and other vertebrates". Comparative Biochemistry and Physiology 39A (1): 125–135. doi:10.1016/0300-9629(71)90352-5. PMID 4399229.
- ↑ Archived March 1, 2009 at the Wayback Machine
- ↑ Archived January 23, 2010 at the Wayback Machine
- ↑ Charlie Sorrel (2007-09-21). "Coca Cola Plans High Tech, Super Cool Sprite". Wired. Condé Nast. Retrieved 2013-12-05.
- ↑ Berendsen, TA; Bruinsma, BG; Puts, CF; Saeidi, N; Usta, OB; Uygun, BE; Izamis, Maria-Louisa; Toner, Mehmet; Yarmush, Martin L; Uygun, Korkut (2014). "Supercooling enables long-term transplantation survival following 4 days of liver preservation". Nature Medecine 20 (7): 790–3. doi:10.1038/nm.3588.
- ↑ Hunka, George (2015-05-06). "A "super cool" way to deliver drugs". R&D. Retrieved May 2015.
Further reading
- Debenedetti, P. G.; Stanley, H. E. (2003). "Supercooled and Glassy Water" (PDF). Physics Today 56 (6): 40–46. Bibcode:2003PhT....56f..40D. doi:10.1063/1.1595053.
- Giovambattista, N.; Angell, C. A.; Sciortino, F.; Stanley, H. E. (July 2004). "Glass-Transition Temperature of Water: A Simulation Study" (PDF). Physical Review Letters 93 (4): 047801. arXiv:cond-mat/0403133. Bibcode:2004PhRvL..93d7801G. doi:10.1103/PhysRevLett.93.047801. PMID 15323794.
- Rogerson, M. A.; Cardoso, S. S. S. (April 2004). "Solidification in heat packs: III. Metallic trigger". AIChE Journal 49 (2): 522–529. doi:10.1002/aic.690490222. Cite uses deprecated parameter
|coauthors=(help)
External links
- Supercooled water and coke on YouTube
- Supercooled water on YouTube
- Super Cooled Water #2 on YouTube
- Supercooled Water Nucleation Experiments on YouTube
- Supercooled liquids on arxiv.org
- Radiolab podcast on supercooling
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
|