Sulfasalazine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
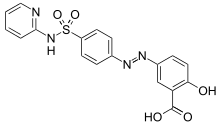
2-hydroxy-5-[(E)-2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid | |
| Clinical data | |
| Trade names | Azulfidine, Salazopyrin, Sulazine, others |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682204 |
| Pregnancy category |
|
| Routes of administration | oral |
| Pharmacokinetic data | |
| Bioavailability | <15% |
| Biological half-life | 5-10 hours |
| Identifiers | |
| CAS Number |
599-79-1 |
| ATC code | A07EC01 |
| PubChem | CID 5384001 |
| DrugBank |
DB00795 |
| ChemSpider |
10481900 |
| UNII |
3XC8GUZ6CB |
| KEGG |
D00448 |
| ChEMBL |
CHEMBL421 |
| Chemical data | |
| Formula | C18H14N4O5S |
| Molar mass | 398.394 g/mol |
| |
| |
| | |
Sulfasalazine (SSZ), marketed under the trade name Azulfidine among others, is a medication used to treat rheumatoid arthritis. It was believed at the time that bacterial infections were the cause of rheumatoid arthritis. Sulfasalazine is a sulfa drug, a derivative of mesalazine, and is formed by combining sulfapyridine and salicylate with an azo coupling.
It was developed in the 1950s.[2] It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[3]
Medical uses
Sulfasalazine is used in the treatment of inflammatory bowel disease, including ulcerative colitis and Crohn's disease. It is also indicated for use in rheumatoid arthritis and used in other types of inflammatory arthritis (e.g. psoriatic arthritis) where it has a beneficial effect. It is often well tolerated compared to other DMARDs.
In clinical trials for the treatment of chronic alcoholics, sulfasalazine has been found to reverse the scarring associated with liver cirrhosis. A study at Newcastle University found that the drug may act to aid the healing of cirrhosis of the liver.[4][5] Cells called myofibroblasts, which contribute to scar tissue in a diseased liver, also appear to secrete proteins that prevent the breakdown of the scar tissue. Sulfasalazine appears to retard this secretion.
It is usually not given to children under 2 years of age.
The use of sulfasalazine in inflammatory bowel disease has declined due mainly to the fact that it yields the metabolite sulfapyridine which gives rise to side-effects such as agranulocytosis and hypospermia. However, the other metabolite of sulfasalazine, 5-aminosalicylic acid (5-ASA) is credited with causing the drug's therapeutic effect. Therefore, 5-ASA and other derivatives of 5-ASA, are now usually preferred and given alone (as mesalazine), despite their increased cost, due to their more favourable side-effect profile.
Sulfasalazine has also been used successfully to treat cases of idiopathic urticaria that do not respond to antihistamines.[6]
Side effects
Sulfasalazine metabolizes to sulfapyridine. Serum levels should be monitored every three months, and more frequently at the outset. Serum levels above 50 μg/l are associated with side effects. In rare cases, Sulfasalazine can cause severe depression in young males. It can also cause temporary infertility. Immune thrombocytopenia has been reported.[7]
Sulfasalazine inhibits dihydropteroate synthase, and can cause folate deficiency and megaloblastic anemia.[8][9][10]
Sulfasalazine can cause hemolytic anemia in people with G6PD deficiency.[11]
Sulfasalazine may cause stomach upset, nausea, vomiting, loss of appetite, headache, dizziness, or unusual tiredness. Skin and urine can become orange, with occasional allergic reactions.[12]
Mechanism of action
Sulfasalazine, and its metabolite 5-ASA, are poorly absorbed from the small intestine. Its main mode of action is therefore believed to be inside the intestine.Approximately one third of a dose of sulfasalazine is absorbed from the small intestine. The remaining two thirds passes into the colon where it is split by bacteria into 5-ASA and SP. SP is well absorbed from the colon (estimated bioavailability 60%); 5-ASA is less well absorbed (estimated bioavailability 10% to 30%).
Bowel disease
In Crohn's disease and ulcerative colitis, it is thought to be an antinflammatory drug that provides topical relief inside the intestine. It does this via a number of mechanisms such as reducing the synthesis of inflammatory mediators known as eicosanoids and inflammatory cytokines. However, compared to glucocorticoids (another class of drug used in the treatment in inflammatory bowel disease), sulfasalazine is only a mild immunosuppressant.
Arthritis
When treatment for arthritis is successful, pain, joint swelling and stiffness will be reduced and this may slow down or stop the development of joint damage. The precise reasons why sulfasalazine are effective in various forms of arthritis is not clearly understood. However, sulfasalazine is thought to work by inhibiting NF-κB.[13][14]
Because sulfasalazine and its metabolite mesalazine (or 5-aminosalicylic acid (5-ASA)) are poorly absorbed into the bloodstream, it is surprising that the drug is effective against symptoms outside of the intestine. One possible explanation is that, given that ulcerative colitis produces arthritic symptoms, the arthritic symptoms resolved by sulfasalazine are actually a product of unrecognized ulcerative colitis, which is effectively treated with sulfasalazine.
The other metabolite, sulfapyridine, is absorbed into the blood, and is believed to be the source of the side-effects discussed above. It is possible that the sulfapyridine is responsible for some of the anti-arthritic effects of sulfasalazine.
References
- ↑ http://www.drugs.com/pregnancy/sulfasalazine.html
- ↑ "Patient information: Disease modifying antirheumatic drugs (DMARDs) (Beyond the Basics)". UpToDate. May 2012. Retrieved 2012-12-09.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Drug 'may reverse liver disease'". BBC News. 2006-09-26. Retrieved 2010-05-24.
- ↑ Fiona Oakley, Muriel Meso, John P. Iredale, Karen Green, Carylyn J. Marek, Xiaoying Zhou, Michael J. May, Harry Millward-Sadler, Matthew C. Wright, Derek A. Mann (Jan 2005). "Inhibition of inhibitor of κB kinases stimulates hepatic stellate cell apoptosis and accelerated recovery from rat liver fibrosis". Gastroenterology 128 (1): 108–120. doi:10.1053/j.gastro.2004.10.003.
- ↑ McGirt LY, Vasagar K, Gober LM, Saini SS, Beck LA (Oct 2006). "Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine". Arch Dermatol 142 (10): 1337–1342. doi:10.1001/archderm.142.10.1337. PMID 17043190.
- ↑ Cantarini L, Tinazzi I, Biasi D, Fioravanti A, Galeazzi M (June 2007). "Sulfasalazine-induced immune thrombocytopenia". Postgraduate Medical Journal 83 (980): e1. doi:10.1136/pgmj.2006.055194. PMC 2600053. PMID 17551063.
- ↑ Inflammatory Bowel Disease~workup at eMedicine
- ↑ Women With Autoimmune Diseases: Medications During Pregnancy and Lactation: Sulfasalazine; http://www.medscape.org/viewarticle/720225_7
- ↑ Hernández-Díaz, Sonia; Werler, Martha M.; Walker, Alexander M.; Mitchell, Allen A. (2000). "Folic Acid Antagonists during Pregnancy and the Risk of Birth Defects". New England Journal of Medicine 343 (22): 1608–14. doi:10.1056/NEJM200011303432204. PMID 11096168.
- ↑ "SulfaSALAzine: Drug Information Provided by Lexi-Comp". Merck & Co., Inc. Jan 2012. Retrieved 2012-07-28.
- ↑ "DRUGS & MEDICATIONS Sulfasalazine". WebMD. WebMD.
- ↑ Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. Journal of Clinical Investigation. 1998;101(5):1163-1174.
- ↑ Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000 Nov;119(5):1209-18.
External links
- Web MD
- Upjohn FDA Label
- Optimal Dosing of 5-Aminosalicylic Acid: 5 Decades of Choosing Between Politicians
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||