Sublimation (phase transition)

Sublimation is the transition of a substance directly from the solid to the gas phase without passing through the intermediate liquid phase.[1] Sublimation is an endothermic phase transition that occurs at temperatures and pressures below a substance's triple point in its phase diagram. The reverse process of sublimation is desublimation or deposition, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe phase changes between solid and gas that avoid the liquid state without specifying the direction of the transition.[2]
At normal pressures, most chemical compounds and elements possess three different states at different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state. The pressure referred to is the partial pressure of the substance, not the total (e.g. atmospheric) pressure of the entire system. So, all solids that possess an appreciable vapor pressure at a certain temperature usually can sublimate in air (e.g. water ice just below 0 °C). For some substances, such as carbon and arsenic, sublimation is much easier than evaporation from the melt, because the pressure of their triple point is very high, and it is difficult to obtain them as liquids.
The term sublimation refers to a physical change of state and is not used to describe transformation of a solid to a gas in a chemical reaction. For example, the dissociation on heating of solid ammonium chloride into hydrogen chloride and ammonia is not sublimation but rather a chemical reaction. Similarly the combustion of candles, containing paraffin wax, to carbon dioxide and water vapor is not sublimation but rather a chemical reaction with oxygen.
Sublimation requires additional energy and is an endothermic change. The enthalpy of sublimation (also called heat of sublimation) can be calculated as the enthalpy of fusion plus the enthalpy of vaporization.
Examples

Carbon dioxide

Solid carbon dioxide (dry ice) sublimates everywhere along the line below the triple point (e.g., at the temperature of −78.5 °C (194.65 K, −104.2 °F) at atmospheric pressure), whereas its melting into liquid CO2 can occur only along the line at pressures and temperatures above the triple point (i.e., 5.2 atm, −56.4 °C).
Water
Snow and ice sublimate, although more slowly, at temperatures below the freezing/melting point temperature line at 0 °C for most pressures; see line below triple point.[3] In freeze-drying, the material to be dehydrated is frozen and its water is allowed to sublimate under reduced pressure or vacuum. The loss of snow from a snowfield during a cold spell is often caused by sunshine acting directly on the upper layers of the snow. Ablation is a process that includes sublimation and erosive wear of glacier ice.
Naphthalene
Naphthalene, an organic compound commonly found in pesticide such as mothball also sublimes. It sublimes easily because it is made of non-polar molecules and has van der Waals intermolecular forces. Naphthalene is a solid that sublimes at standard atmospheric temperature[4] with the sublimation point at around 80˚C or 176˚F.[5] At low temperature, its vapour pressure is high enough, 1 mmHg at 53˚C,[6] to make the solid form of naphthalene evaporate into gas. On the cool surface, the sublimated vapour will be solidified to form a needle-like crystal.


Other substances

Iodine produces fumes on gentle heating. It is possible to obtain liquid iodine at atmospheric pressure by controlling the temperature at just above the melting point of iodine. In forensic science, iodine vapor can reveal latent fingerprints on paper.[7] Arsenic can also sublimate at high temperatures.
Sublimation purification

Sublimation is a technique used by chemists to purify compounds. A solid is typically placed in a sublimation apparatus and heated under vacuum. Under this reduced pressure, the solid volatilizes and condenses as a purified compound on a cooled surface (cold finger), leaving a non-volatile residue of impurities behind. Once heating ceases and the vacuum is removed, the purified compound may be collected from the cooling surface.[8][9] For even higher purification efficiencies a temperature gradient is applied, which also allows for the separation of different fractions. Typical setups use an evacuated glass tube that is gradually heated in a controlled manner. The material flow is from the hot end, where the initial material is placed, to the cold end that is connected to a pump stand. By controlling temperatures along the length of the tube the operator can control the zones of recondensation, with very volatile compounds being pumped out of the system completely (or caught by a separate cold trap), moderately volatile compounds recondensating along the tube according to their different volatilities, and non-volatile compounds remaining in the hot end. Vacuum sublimation of this type is also the method of choice for purification of organic compounds for the use in the organic electronics industry, where very high purities (often > 99.99%) are needed to satisfy the standards for consumer electronics and other applications.
Historical usage
In ancient alchemy, a protoscience that contributed to the development of modern chemistry and medicine, alchemists developed a structure of basic laboratory techniques, theory, terminology, and experimental methods. Sublimation was used to refer to the process in which a substance is heated to a vapor, then immediately collects as sediment on the upper portion and neck of the heating medium (typically a retort or alembic), but can also be used to describe other similar non-laboratory transitions. It is mentioned by alchemical authors such as Basil Valentine and George Ripley, and in the Rosarium philosophorum, as a process necessary for the completion of the magnum opus. Here, the word sublimation is used to describe an exchange of "bodies" and "spirits" similar to laboratory phase transition between solids and gases. Valentine, in his Triumphal Chariot of Antimony (published 1678) makes a comparison to spagyrics in which a vegetable sublimation can be used to separate the spirits in wine and beer.[10] Ripley uses language more indicative of the mystical implications of sublimation, indicating that the process has a double aspect in the spiritualization of the body and the corporalizing of the spirit.[11] He writes:[12]
And Sublimations we make for three causes,
The first cause is to make the body spiritual.
The second is that the spirit may be corporeal,
And become fixed with it and consubstantial.
The third cause is that from its filthy original.
It may be cleansed, and its saltiness sulphurious
May be diminished in it, which is infectious.
Sublimation predictions
The enthalpy of sublimation has commonly been predicted using the equipartition theorem. If the lattice energy is assumed to be approximately half the packing energy, then the following thermodynamic corrections can be applied to predict the enthalpy of sublimation. Assuming a 1 molar ideal gas gives a correction for the thermodynamic environment (pressure and volume) in which pV = RT, hence a correction of 1RT. Additional corrections for the vibrations, rotations and translation then need to be applied. From the equipartition theorem gaseous rotation and translation contribute 1.5RT each to the final state, therefore a +3RT correction. Crystalline vibrations and rotations contribute 3RT each to the initial state, hence −6RT. Summing the RT corrections ; −6RT + 3RT + RT = −2RT.[13] This leads to the following approximate sublimation enthalpy. A similar approximation can be found for the entropy term if rigid bodies are assumed.[14]
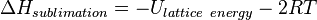
See also
- Ablation
- Dye-sublimation printer, Freezer burn – common processes involving sublimation
- Enthalpy of sublimation
- Phase diagram
| To | |||||
|---|---|---|---|---|---|
| Solid | Liquid | Gas | Plasma | ||
| From | Solid | Solid-solid transformation | Melting | Sublimation | — |
| Liquid | Freezing | — | Boiling / evaporation | — | |
| Gas | Deposition | Condensation | — | Ionization | |
| Plasma | — | — | Recombination / deionization | — | |
References
- ↑ Sublimate. Merriam-webster.com. Retrieved on 2015-11-25.
- ↑ Sublime. Dictionary.reference.com. Retrieved on 2015-11-25.
- ↑ Fassnacht, S. R. (2004). "Estimating Alter-shielded gauge snowfall undercatch, snowpack sublimation, and blowing snow transport at six sites in the coterminous USA" (PDF). Hydrol. Process. 18: 3481–3492. doi:10.1002/hyp.5806.
- ↑ Caroll, J. (2014). Natural Gas Hydrates. p. 16. ISBN 9780128005750.
- ↑ Staff writer(s) (2015). "what solid go through sublimation?". National Science Foundation and UCSB School-University partnership. Retrieved 13 November 2015.
- ↑ Pavia. D. (2005). Introduction to organic laboratory technique. pp. 781–782. ISBN 0534408338.
- ↑ James Girard (2011). Criminalistics: Forensic Science, Crime and Terrorism. Jones & Bartlett Learning. pp. 143–144. ISBN 0-7637-7731-5.
- ↑ R. B. King Organometallic Syntheses. Volume 1 Transition-Metal Compounds; Academic Press: New York, 1965. ISBN 0-444-42607-8.
- ↑ Laurence M. Harwood, Christopher J. Moody (1989). Experimental organic chemistry: Principles and Practice (Illustrated ed.). WileyBlackwell. pp. 154–155. ISBN 0-632-02017-2.
- ↑ Francis Barrett. The lives of alchemystical philosophers: with a critical catalogue of books in occult chemistry, and a selection of the most celebrated treatises on the theory and practice of the hermetic art. Printed by Macdonald and son, for Lackington, Allen, & co., 1815. p. 233
- ↑ DiBernard, Barbara (1980). Alchemy and Finnegans wake. SUNY Press, p. 57, ISBN 0873953886.
- ↑ Ripley, George (1591). Compound of Alchemy.
- ↑ Gavezzotti, A (1997). Theoretical Aspects and Computer Modeling of the Molecular Solid State. Chichester: Wiley and Sons.
- ↑ McDonagh, J. L.; Nath; De Ferrari, Luna; Van Mourik, Tanja; Mitchell, John B. O. (2014). "Uniting Cheminformatics and Chemical Theory To Predict the Intrinsic Aqueous Solubility of Crystalline Druglike Molecules". Journal of Chemical Information and Modeling 54 (3): 844. doi:10.1021/ci4005805.
| ||||||||||||||||||||||||||||||||||||

