Strontian process
The strontian process is an obsolete chemical method to recover sugar from molasses. Its use in Europe peaked in the middle of the 19th century. The name strontian comes from the Scottish village Strontian where the source mineral strontianite (strontium carbonate) was first found.
Chemistry
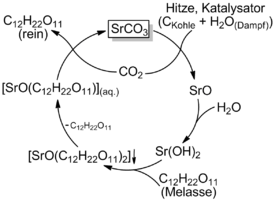
Strontium carbonate is a recycled coreactant in this process.
- Strontium carbonate is calcined with carbon in the presence of steam to form strontium hydroxide. The strontium and carbon dioxide formed are rejoined later in the process, forming strontium carbonate once again.[1]
- SrCO3 + C + H2O = Sr(OH)2 + CO2
- In a molasses solution kept near 100 °C,[2] the hydroxide reacts with soluble sugars to form water and the poorly soluble strontium-saccharate which is filtered out, but kept awash in near-boiling water.
- Sr(OH)2 + 2C12H22O11 = SrO(C12H22O11)2 + H2O
- The saccharate liquid is cooled to 10 °C, cracking off one of the sugars
- SrO(C12H22O11)2 = SrO(C12H22O11) + C12H22O11
- The carbon dioxide (from the calcination) is bubbled through the saccharate solution, cracking off the second sugar and reforming the strontium carbonate, which is filtered off.
- SrO(C12H22O11) + CO2 = SrCO3 + C12H22O11
- The sugar is then extracted through evaporating the remaining solution.
There are two types of strontium-saccharide: one at low temperature, the strontium-monosaccharide; and the second at high temperature, the strontium-disaccharide.[3]
History
Molasses is a by-product of sugar production from sugar beet, and it is contains more than 50% sugar itself. The French chemists Hippolyte Leplay and Augustin-Pierre Dubrunfaut developed a process for extracting sugar from molasses, reacting them with barium oxide, to give the insoluble barium-saccharates.[4] In 1849, they expanded their patent to include strontium salts. Apparently, this patent application had the only purpose to legally secure the so-called baryte process, since the strontian process from Leplay and Dubrunfaut probably wouldn't work as described.[5]
Only later, through the works of Carl Scheibler, was it possible to apply the strontian process in an industrial basis.[6] According to Scheibler the procedure must be carried out at boiling temperatures.
Repercussion in Germany
The Scheibler procedure came into use in the Dessauer Sugar Refinery (in Dessau), through Emil Fleischer. In the region called Münsterland, its arrival caused a ″gold fever″ breakout, regarding the strontianite mining.[7] One of the biggest mines, at Drensteinfurt, was named after Dr. Reichardt, the director of the Dessauer Sugar Refinery. A further place the strontian process came to be used was the Sugar Factory Rositz (in Rositz).
Yet by 1883, the demand on strontianite had begun to shrink. Firstly, it was replaced by another strontium mineral (celestine), that could be imported from England, in a cheaper way. Secondly, the prices for sugar decreased so much, that the production from molasses was no longer worthwhile.
Literature (further reading)
- Börnchen, Martin : Strontianit, Exhibition guide from the University Library of the Free University of Berlin, 2005 (PDF; 6,5 MB). In German.
- Heriot, T. H. P.: The Manufacture of Sugar from the Cane and Beet, Green and Company, 1920, pp. 341–342 (archive online).
- Krause, G.: Der Schiedsspruch in Sachen des Scheibler'schen Monostrontiumsaccharat-Patentes, Chemiker Zeitung, nr. 32, 19th April, 1885, (PDF; 4,94 MB). In German.
References
- ↑ Reinhard Brauns, Das Mineralreich, vol. 1, Fritz Lehmann publishing, Stuttgart (1903), pp. 402-403. (German).
- ↑ The separation of sugar by the Strontian method
- ↑ Dinglers Polytechnisches Journal (COMPILATION) (in German) 248. 1883. pp. 426–428.
- ↑ J. Nicklès (1854). "Leplay's Verfahren zur Abscheidung des krystallisirbaren Zuckers aus der Melasse".
Dinglers Polytechnisches Journal (Compilation) (in German) 131 (XVII): 47–50. - ↑ De Indische opmerker, 15 March 1883 (PDF; 8,08 MB) (Dutch).
- ↑ University of Arizona, Bulletin No. 35, (1916-1917) (PDF; 2,3 MB)
- ↑ Martin Börnchen: Der Strontianitbergbau im Münsterland (PDF; 4,3 MB). (German).