Streptomycin
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
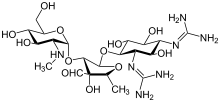
5-(2,4-diguanidino- 3,5,6-trihydroxy-cyclohexoxy)- 4-[4,5-dihydroxy-6-(hydroxymethyl) -3-methylamino-tetrahydropyran-2-yl] oxy-3-hydroxy-2-methyl -tetrahydrofuran-3-carbaldehyde | |
| Clinical data | |
| AHFS/Drugs.com | monograph |
| Pregnancy category |
|
| Legal status | |
| Routes of administration | Intramuscular, intravenous |
| Pharmacokinetic data | |
| Bioavailability | 84% to 88% (est.)[1] |
| Biological half-life | 5 to 6 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
57-92-1 |
| ATC code | A07AA04 J01GA01 |
| PubChem | CID 19649 |
| DrugBank |
DB01082 |
| ChemSpider |
18508 |
| UNII |
Y45QSO73OB |
| KEGG |
D08531 |
| ChEBI |
CHEBI:17076 |
| ChEMBL |
CHEMBL1201194 |
| NIAID ChemDB | 07346 |
| Chemical data | |
| Formula | C21H39N7O12 |
| Molar mass | 581.574 g/mol |
| |
| |
| Physical data | |
| Melting point | 12 °C (54 °F) |
| | |
Streptomycin is an antibiotic (antimycobacterial) drug, the first of a class of drugs called aminoglycosides to be discovered, and it was the first effective treatment for tuberculosis. It is derived from the actinobacterium Streptomyces griseus. Streptomycin is a bactericidal antibiotic.[2] Adverse effects of this medicine are ototoxicity, nephrotoxicity, fetal auditory toxicity, and neuromuscular paralysis.
It is on the World Health Organization's List of Essential Medicines, the most important medication needed in a basic health system.[3]
Uses
Treatment of diseases
- Infective endocarditis caused by enterococcus when the organism is not sensitive to Gentamicin
- Tuberculosis in combination with other anti-TB drugs. It is not the first-line treatment, except in medically under-served populations where the cost of more expensive treatments is prohibitive.
- Plague (Yersinia pestis) has historically been treated with it as the first-line treatment. However It is approved for this purpose only by the U.S. Food and Drug Administration.
- In veterinary medicine, streptomycin is the first-line antibiotic for use against gram negative bacteria in large animals (horses, cattle, sheep, etc.). It is commonly combined with procaine penicillin for intramuscular injection.
- Tularemia infections have been treated mostly with Streptomycin and some other antibiotic drugs, which is effective, but the infection is resistant to Penicillin.
While streptomycin traditionally is given intramuscularly (indeed, in many countries it is only licensed to be used intramuscularly), the drug may also be administered intravenously.[1]
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Streptomycin and other antibacterial drugs, Streptomycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Pesticide and fungicide
Streptomycin also is used as a pesticide, to combat the growth of bacteria, fungi, and algae. Streptomycin controls bacterial and fungal diseases of certain fruit, vegetables, seed, and ornamental crops, and it controls algae in ornamental ponds and aquaria. A major use is in the control of fireblight on apple and pear trees. As in medical applications, extensive use can be associated with the development of resistant strains.
Cell culture
Streptomycin, in combination with penicillin, is used in a standard antibiotic cocktail to prevent bacterial infection in cell culture. STBL3, a strain of bacteria commonly used in laboratories for growth of unstable plasmid DNA, i.e. Lentiviral plasmids containing LTR, are resistant to Streptomycin.
Protein purification
When purifying protein from a biological extract, streptomycin sulfate is sometimes added as a means of removing nucleic acids. Since it binds to ribosomes and precipitates out of solution, it serves as a method for removing rRNA, mRNA, and even DNA if the extract is from a prokaryote.
Spectrum of activity
Streptomycin can be used clinically to treat tuberculosis in combination with other medications and susceptible strains which cause bacterial endocarditis. The following represents MIC susceptibility for a few medically significant microorganisms.
- Mycobacterium tuberculosis: 1 μg/ml - 2 μg/ml
- Staphylococcus aureus: 4 μg/ml
Side effects
Fever and rashes result from persistent use. The Vestibular portion of cranial nerve VIII (the vestibulococlear nerve) can be affected, resulting in tinnitus, vertigo and ataxia. It can also lead to nephrotoxicity and can potentially interfere with diagnosis of kidney malfunctioning.[6]
Mechanism of action
Streptomycin is a protein synthesis inhibitor. It binds to the small 16S rRNA of the 30S subunit of the bacterial ribosome, interfering with the binding of formyl-methionyl-tRNA to the 30S subunit.[7] This leads to codon misreading, eventual inhibition of protein synthesis and ultimately death of microbial cells through mechanisms that are still not understood. Speculation on this mechanism indicates that the binding of the molecule to the 30S subunit interferes with 50S subunit association with the mRNA strand. This results in an unstable ribosomal-mRNA complex, leading to a frameshift mutation and defective protein synthesis; leading to cell death.[8] Humans have ribosomes which are structurally different from those in bacteria, so the drug does not have this effect in human cells. At low concentrations, however, streptomycin only inhibits growth of the bacteria by inducing prokaryotic ribosomes to misread mRNA.[9] Streptomycin is an antibiotic that inhibits both Gram-positive and Gram-negative bacteria,[10] and is therefore a useful broad-spectrum antibiotic.
History
Streptomycin was first isolated on October 19, 1943, by Albert Schatz, a graduate student, in the laboratory of Selman Abraham Waksman at Rutgers University in a research project funded by Merck and Co.[11][12] Dr. Waksman and his laboratory staff discovered several antibiotics, including actinomycin, clavacin, streptothricin, streptomycin, grisein, neomycin, fradicin, candicidin, and candidin. Of these, streptomycin and neomycin found extensive application in the treatment of numerous infectious diseases. Streptomycin was the first antibiotic cure for tuberculosis (TB).
At the end of World War II, the United States Army experimented with streptomycin to treat life-threatening infections at a military hospital in Battle Creek, Michigan. The first patient treated did not survive; the second patient survived but became blind as a side effect of the treatment. In March 1946, the third patient—Robert J. Dole, later Majority Leader of the United States Senate and Presidential nominee—experienced a rapid and robust recovery.[13]
The first randomized trial of streptomycin against pulmonary tuberculosis was carried out in 1946–1947 by the MRC Tuberculosis Research Unit under the chairmanship of Sir Geoffrey Marshall (1887–1982). The trial was both double-blind and placebo-controlled. It is widely accepted to have been the first randomised curative trial.[14]
Results showed efficacy against TB, albeit with minor toxicity and acquired bacterial resistance to the drug.[15]
See also
- Philip D'Arcy Hart - The British medical researcher and pioneer in tuberculosis treatment in the early twentieth century.
References
- 1 2 Zhu M, Burman WJ, Jaresko GS, Berning SE, Jelliffe RW, Peloquin CA. (October 2001). "Population pharmacokinetics of intravenous and intramuscular streptomycin in patients with tuberculosis". Pharmacother. 21 (9): 1037–1045. doi:10.1592/phco.21.13.1037.34625. PMID 11560193. Retrieved 2010-05-25.
- ↑ Singh B, Mitchison DA (16 January 1954). "Bactericidal Activity of Streptomycin and Isoniazid Against Tubercle Bacilli". British Medical Journal 1 (4854): 130–132. doi:10.1136/bmj.1.4854.130. PMC 2084433. PMID 13106497.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ http://antibiotics.toku-e.com/antimicrobial_1099_1.html
- ↑ http://www.toku-e.com/Assets/MIC/Streptomycin%20sulfate.pdf
- ↑ Syal K, Srinivasan A & Banerjee D Streptomycin interference in Jaffe reaction — Possible false positive creatinine estimation in excessive dose exposure. Clinical Biochemistry 46, 177-179, 2013
- ↑ Sharma D, Cukras AR, Rogers EJ, Southworth DR, Green R (7 December 2007). "Mutational analysis of S12 protein and implications for the accuracy of decoding by the ribosome". Journal of Molecular Biology 374 (4): 1065–76. doi:10.1016/j.jmb.2007.10.003. PMC 2200631. PMID 17967466.
- ↑ Raymon, Lionel P. (2011). COMLEX Level 1 Pharmacology Lecture Notes. Miami, FL: Kaplan, Inc. p. 181. CM4024K.
- ↑ Voet, Donald & Voet, Judith G. (2004). Biochemistry (3rd ed.). John Wiley & Sons. p. 1341. ISBN 0-471-19350-X.
- ↑ Jan-Thorsten Schantz; Kee-Woei Ng (2004). A manual for primary human cell culture. World Scientific. p. 89.
- ↑ Comroe JH Jr (1978). "Pay dirt: the story of streptomycin. Part I: from Waksman to Waksman". American Review of Respiratory Disease 117 (4): 773–781. PMID 417651.
- ↑ Kingston W (July 2004). "Streptomycin, Schatz v. Waksman, and the balance of credit for discovery". J Hist Med Allied Sci 59 (3): 441–62. doi:10.1093/jhmas/jrh091. PMID 15270337.
- ↑ Cramer, Richard Ben, What It Takes (New York, 1992), pp. 110-11.
- ↑ Metcalfe NH (February 2011). "Sir Geoffrey Marshall (1887-1982): respiratory physician, catalyst for anaesthesia development, doctor to both Prime Minister and King, and World War I Barge Commander". J Med Biogr 19 (1): 10–4. doi:10.1258/jmb.2010.010019. PMID 21350072.
- ↑ D'Arcy Hart P (August 1999). "A change in scientific approach: from alternation to randomised allocation in clinical trials in the 1940s". British Medical Journal 319 (7209): 572–3. doi:10.1136/bmj.319.7209.572. PMC 1116443. PMID 10463905.
Further reading
- "Notebooks Shed Light on an Antibiotic’s Contested Discovery," New York Times, June 12, 2012, by Peter Pringle
- Streptomycin bound to proteins in the PDB
- Kingston, William (2004). "Streptomycin, Schatz v. Waksman, and the Balance of Credit for Discovery". Journal of the History of Medicine and Allied Sciences 59 (3): 441–462. doi:10.1093/jhmas/jrh091. PMID 15270337.
- Mistiaen, Veronique (2 November 2002). "Time, and the great healer". The Guardian.. The history behind the discovery of streptomycin.
- EPA R.E.D. Facts sheet on use of streptomycin as a pesticide.
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|