Staudinger reaction
| Staudinger reaction | |
|---|---|
| Named after | Hermann Staudinger |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | staudinger-reaction |
| RSC ontology ID | RXNO:0000066 |
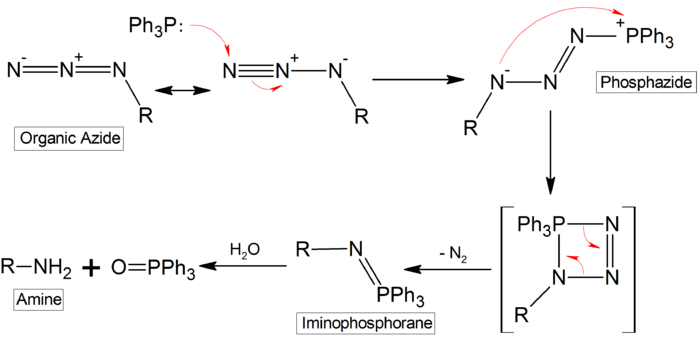
The Staudinger reaction or Staudinger reduction is a chemical reaction in which the combination of an azide with a phosphine or phosphite produces an iminophosphorane intermediate.[1][2] Combined with the hydrolysis of the aza-ylide to produce a phosphine oxide and an amine, this reaction is a mild method of reducing an azide to an amine. Triphenylphosphine is commonly used as the reducing agent, yielding triphenylphosphine oxide as the side product in addition to the amine.
The reaction was discovered by and named after Hermann Staudinger.
An example of a Staudinger reduction is the organic synthesis of this pinwheel compound:[3]
Reaction mechanism
The reaction mechanism centers around the formation of an iminophosphorane through nucleophilic addition of the phosphine at the terminal nitrogen atom of the azide and expulsion of nitrogen. This intermediate is then hydrolyzed in the second step to the amine and triphenylphosphine oxide
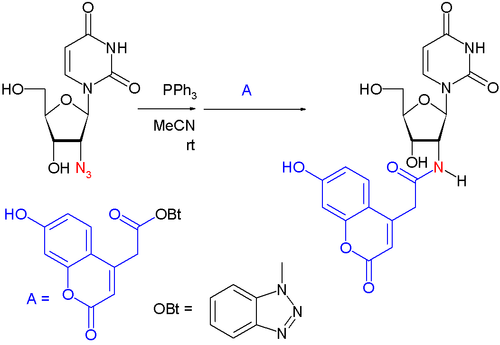
Staudinger ligation
Developed by Saxon and Bertozzi in 2000, the Staudinger ligation is a modification of the classical Staudinger reaction in which an electrophilic trap (usually a methyl ester) is placed on the triaryl phosphine.[4] In aqueous media, the aza-ylide intermediate rearranges, to produce an amide linkage and the phosphine oxide, and is so named the Staudinger ligation because it ligates two molecules together, whereas in the classical Staudinger reaction, the two products are not covalently linked after hydrolysis. A traceless version of the reaction leaves behind no residual atoms.[5]
Applications
The Staudinger ligation has seen many applications in the field of chemical biology.
In one application this reaction is used to create a bond between a nucleoside and a fluorescent marker:[6][7]
References
- ↑ Staudinger, H.; Meyer, J. (1919), "Über neue organische Phosphorverbindungen III. Phosphinmethylenderivate und Phosphinimine", Helv. Chim. Acta 2 (1): 635, doi:10.1002/hlca.19190020164
- ↑ Gololobov, Y. G. (1981), "Sixty years of staudinger reaction", Tetrahedron 37 (3): 437, doi:10.1016/S0040-4020(01)92417-2
- ↑ Karl J. Wallace, Robert Hanes, Eric Anslyn, Jeroni Morey, Kathleen V. Kilway, Jay Siegeld (2005), "Preparation of 1,3,5-Tris(aminomethyl)-2,4,6-triethylbenzene from Two Versatile 1,3,5-Tri(halosubstituted) 2,4,6-Triethylbenzene Derivatives", Synthesis 2005 (12): 2080, doi:10.1055/s-2005-869963
- ↑ Saxon, E.; Bertozzi, C. R. (2000), "Cell Surface Engineering by a Modified Staudinger Reaction", Science 287 (5460): 2007, Bibcode:2000Sci...287.2007S, doi:10.1126/science.287.5460.2007, PMID 10720325.
- ↑ Nilsson, B. L.; Kiessling, L. L.; Raines, R. T. (2000). "Staudinger ligation: A peptide from a thioester and azide". Org. Lett. 2: 1939–1941. doi:10.1021/ol0060174. PMID 10891196.
- ↑ Kosiova I, Janicova A, Kois P (2006), "Synthesis of coumarin or ferrocene labeled nucleosides via Staudinger ligation", Beilstein Journal of Organic Chemistry 2 (1): 23, doi:10.1186/1860-5397-2-23, PMC 1779791, PMID 17137496.
- ↑ the nucleoside is based on deoxyuridine, the marker is a coumarin with a carboxyl group activated by HOBT
External links
- Staudinger Reaction at organic-chemistry.org accessed 060906.
- Julia-Staudinger Reaction