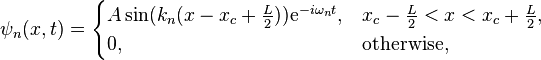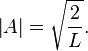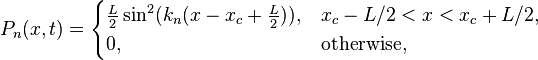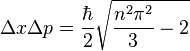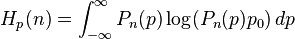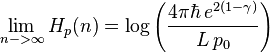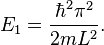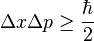Particle in a box

In quantum mechanics, the particle in a box model (also known as the infinite potential well or the infinite square well) describes a particle free to move in a small space surrounded by impenetrable barriers. The model is mainly used as a hypothetical example to illustrate the differences between classical and quantum systems. In classical systems, for example a ball trapped inside a large box, the particle can move at any speed within the box and it is no more likely to be found at one position than another. However, when the well becomes very narrow (on the scale of a few nanometers), quantum effects become important. The particle may only occupy certain positive energy levels. Likewise, it can never have zero energy, meaning that the particle can never "sit still". Additionally, it is more likely to be found at certain positions than at others, depending on its energy level. The particle may never be detected at certain positions, known as spatial nodes.
The particle in a box model provides one of the very few problems in quantum mechanics which can be solved analytically, without approximations. This means that the observable properties of the particle (such as its energy and position) are related to the mass of the particle and the width of the well by simple mathematical expressions. Due to its simplicity, the model allows insight into quantum effects without the need for complicated mathematics. It is one of the first quantum mechanics problems taught in undergraduate physics courses, and it is commonly used as an approximation for more complicated quantum systems.
One-dimensional solution

The simplest form of the particle in a box model considers a one-dimensional system. Here, the particle may only move backwards and forwards along a straight line with impenetrable barriers at either end.[1] The walls of a one-dimensional box may be visualised as regions of space with an infinitely large potential energy. Conversely, the interior of the box has a constant, zero potential energy.[2] This means that no forces act upon the particle inside the box and it can move freely in that region. However, infinitely large forces repel the particle if it touches the walls of the box, preventing it from escaping. The potential energy in this model is given as
where L is the length of the box, xc is the location of the center of the box and x is the position of the particle within the box. Simple cases include the centered box (xc = 0 ) and the shifted box (xc = L/2 )
Position wave function
In quantum mechanics, the wavefunction gives the most fundamental description of the behavior of a particle; the measurable properties of the particle (such as its position, momentum and energy) may all be derived from the wavefunction.[3]
The wavefunction  can be found by solving the Schrödinger equation for the system
can be found by solving the Schrödinger equation for the system
where 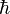 is the reduced Planck constant,
is the reduced Planck constant,  is the mass of the particle,
is the mass of the particle,  is the imaginary unit and
is the imaginary unit and  is time.
is time.
Inside the box, no forces act upon the particle, which means that the part of the wavefunction inside the box oscillates through space and time with the same form as a free particle:[1][4]
-
![\psi(x,t) = [A \sin(kx) + B \cos(kx)]\mathrm{e}^{-i\omega t},](../I/m/7570a117d319bd2b263cfb3bc2c08fbd.png)
(1)
where 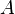 and
and 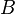 are arbitrary complex numbers. The frequency of the oscillations through space and time are given by the wavenumber
are arbitrary complex numbers. The frequency of the oscillations through space and time are given by the wavenumber  and the angular frequency
and the angular frequency 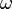 respectively. These are both related to the total energy of the particle by the expression
respectively. These are both related to the total energy of the particle by the expression
which is known as the dispersion relation for a free particle.[1] Here one must notice that now, since the particle is not entirely free but under the influence of a potential (the potential V described above), the energy of the particle given above is not the same thing as 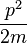 where p is the momentum of the particle, and thus the wavenumber k above actually describes the energy states of the particle, not the momentum states (id est, it turns out that the momentum of the particle is not given by
where p is the momentum of the particle, and thus the wavenumber k above actually describes the energy states of the particle, not the momentum states (id est, it turns out that the momentum of the particle is not given by 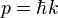 ). In this sense, it is quite dangerous to call the number k a wavenumber, since it is not related to momentum like "wavenumber" usually is. The rationale for calling k the wavenumber is that it enumerates the number of crests that the wavefuntion has inside the box, and in this sense it is a wavenumber. This discrepancy can be seen more clearly below, when we find out that the energy spectrum of the particle is discrete (only discrete values of energy are allowed) but the momentum spectrum is continuous (momentum can vary continuously) and in particular, the relation
). In this sense, it is quite dangerous to call the number k a wavenumber, since it is not related to momentum like "wavenumber" usually is. The rationale for calling k the wavenumber is that it enumerates the number of crests that the wavefuntion has inside the box, and in this sense it is a wavenumber. This discrepancy can be seen more clearly below, when we find out that the energy spectrum of the particle is discrete (only discrete values of energy are allowed) but the momentum spectrum is continuous (momentum can vary continuously) and in particular, the relation 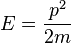 for the energy and momentum of the particle does not hold. As said above, the reason this relation between energy and momentum does not hold is that the particle is not free, but there is a potential V in the system, and the energy of the particle is
for the energy and momentum of the particle does not hold. As said above, the reason this relation between energy and momentum does not hold is that the particle is not free, but there is a potential V in the system, and the energy of the particle is  , where T is the kinetic and V the potential energy.
, where T is the kinetic and V the potential energy.
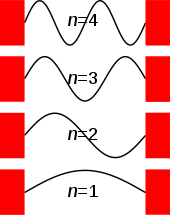
The size (or amplitude) of the wavefunction at a given position is related to the probability of finding a particle there by  . The wavefunction must therefore vanish everywhere beyond the edges of the box.[1][4] Also, the amplitude of the wavefunction may not "jump" abruptly from one point to the next.[1] These two conditions are only satisfied by wavefunctions with the form
. The wavefunction must therefore vanish everywhere beyond the edges of the box.[1][4] Also, the amplitude of the wavefunction may not "jump" abruptly from one point to the next.[1] These two conditions are only satisfied by wavefunctions with the form
where [5]
 ,
,
and
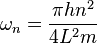 ,
,
where n is a positive integer (1,2,3,4...). For a shifted box (xc = L/2), the solution is particularly simple. The simplest solutions,  or
or  both yield the trivial wavefunction
both yield the trivial wavefunction 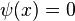 , which describes a particle that does not exist anywhere in the system.[6] Negative values of
, which describes a particle that does not exist anywhere in the system.[6] Negative values of  are neglected, since they give wavefunctions identical to the positive
are neglected, since they give wavefunctions identical to the positive  solutions except for a physically unimportant sign change.[6] Here one sees that only a discrete set of energy values and wavenumbers k are allowed for the particle. Usually in quantum mechanics it is also demanded that the derivative of the wavefunction in addition to the wavefunction itself be continuous; here this demand would lead to the only solution being the constant zero function, which is not what we desire, so we give up this demand (as this system with infinite potential can be regarded as a nonphysical abstract limiting case, we can treat it as such and "bend the rules"). Note that giving up this demand means that the wavefunction is not a differentiable function at the boundary of the box, and thus it can be said that the wavefunction does not solve the Schrödinger equation at the boundary points
solutions except for a physically unimportant sign change.[6] Here one sees that only a discrete set of energy values and wavenumbers k are allowed for the particle. Usually in quantum mechanics it is also demanded that the derivative of the wavefunction in addition to the wavefunction itself be continuous; here this demand would lead to the only solution being the constant zero function, which is not what we desire, so we give up this demand (as this system with infinite potential can be regarded as a nonphysical abstract limiting case, we can treat it as such and "bend the rules"). Note that giving up this demand means that the wavefunction is not a differentiable function at the boundary of the box, and thus it can be said that the wavefunction does not solve the Schrödinger equation at the boundary points 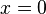 and
and 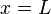 (but does solve everywhere else).
(but does solve everywhere else).
Finally, the unknown constant 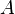 may be found by normalizing the wavefunction so that the total probability density of finding the particle in the system is 1. It follows that
may be found by normalizing the wavefunction so that the total probability density of finding the particle in the system is 1. It follows that
Thus, A may be any complex number with absolute value √(2/L); these different values of A yield the same physical state, so A = √(2/L) can be selected to simplify.
It is expected that the eigenvalues, i.e., the energy 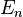 of the box should be the same regardless of its position in space, but
of the box should be the same regardless of its position in space, but 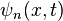 changes. Notice that
changes. Notice that  represents a phase shift in the wave function, This phase shift has no effect when solving the Schrödinger equation, and therefore does not affect the eigenvalue.
represents a phase shift in the wave function, This phase shift has no effect when solving the Schrödinger equation, and therefore does not affect the eigenvalue.
Momentum wave function
The momentum wavefunction is proportional to the Fourier transform of the position wavefunction. With  (note that this k, describing the momentum states, is not the same thing as thekn above, which described the energy states) the momentum wave function is given by:
(note that this k, describing the momentum states, is not the same thing as thekn above, which described the energy states) the momentum wave function is given by:
where sinc(.) is the sinc function (sinc(x)=sin(x)/x). for the centered box (xc = 0), the solution is particularly simple since the phase factor on the right is unity. Note that in the problematic case of  (i.e.
(i.e. 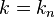 ), the sinc function is also zero, and the limiting case is taken.
), the sinc function is also zero, and the limiting case is taken.
It can be seen that the momentum spectrum is continuous, and one can conclude that for energy state described by the  wavenumber, the momentum can, when measured, attain also other values than
wavenumber, the momentum can, when measured, attain also other values than  , although these are the most likely momentum values (the momentum wavefunction attains its greatest values at these momentum values). Hence one also sees that since the energy is
, although these are the most likely momentum values (the momentum wavefunction attains its greatest values at these momentum values). Hence one also sees that since the energy is 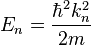 (since we are talking about the n:th energy eigenstate) the relation
(since we are talking about the n:th energy eigenstate) the relation 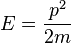 does not necessarily hold; here p is the measured momentum (since the energy eigenstate
does not necessarily hold; here p is the measured momentum (since the energy eigenstate  is not a momentum eigenstate, and actually not even a superposition of two momentum eigenstates, as one could imagine from equation (1) above, it has no well-defined momentum before measurement).
is not a momentum eigenstate, and actually not even a superposition of two momentum eigenstates, as one could imagine from equation (1) above, it has no well-defined momentum before measurement).
Position and momentum probability distributions
In classical physics, the particle can be detected anywhere in the box with equal probability. In quantum mechanics, however, the probability density for finding a particle at a given position is derived from the wavefunction as 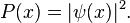 For the particle in a box, the probability density for finding the particle at a given position depends upon its state, and is given by
For the particle in a box, the probability density for finding the particle at a given position depends upon its state, and is given by
Thus, for any value of n greater than one, there are regions within the box for which  , indicating that spatial nodes exist at which the particle cannot be found.
, indicating that spatial nodes exist at which the particle cannot be found.
In quantum mechanics, the average, or expectation value of the position of a particle is given by
For the steady state particle in a box, it can be shown that the average position is always 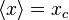 , regardless of the state of the particle. For a superposition of states, the expectation value of the position will change based on the cross term which is proportional to
, regardless of the state of the particle. For a superposition of states, the expectation value of the position will change based on the cross term which is proportional to  .
.
The variance in the position is a measure of the uncertainty in position of the particle:
The probability density for finding a particle with a given momentum is derived from the wavefunction as  . As with position, the probability density for finding the particle at a given momentum depends upon its state, and is given by
. As with position, the probability density for finding the particle at a given momentum depends upon its state, and is given by
where, again,  . The expectation value for the momentum is then calculated to be zero, and the variance in the momentum is calculated to be:
. The expectation value for the momentum is then calculated to be zero, and the variance in the momentum is calculated to be:
The uncertainties in position and momentum (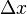 and
and  ) are defined as being equal to the square root of their respective variances, so that:
) are defined as being equal to the square root of their respective variances, so that:
This product increases with increasing n, having a minimum value for n=1. The value of this product for n=1 is about equal to 0.568 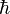 which obeys the Heisenberg uncertainty principle, which states that the product will be greater than or equal to
which obeys the Heisenberg uncertainty principle, which states that the product will be greater than or equal to 
Another measure of uncertainty in position is the information entropy of the probability distribution Hx:[7]
where x0 is an arbitrary reference length.
Another measure of uncertainty in momentum is the information entropy of the probability distribution Hp:
where p0 is an arbitrary reference momentum. The integral is difficult to express analytically for general n, but in the limit as n approaches infinity:[7][8]
where γ is Euler's constant. The quantum mechanical entropic uncertainty principle states that for 
 (nats)
(nats)
For  , the sum of the position and momentum entropies yields:
, the sum of the position and momentum entropies yields:
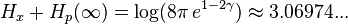 (nats)
(nats)
which satisfies the quantum entropic uncertainty principle.
Energy levels

The energies which correspond with each of the permitted wavenumbers may be written as[5]
 .
.
The energy levels increase with  , meaning that high energy levels are separated from each other by a greater amount than low energy levels are. The lowest possible energy for the particle (its zero-point energy) is found in state 1, which is given by[9]
, meaning that high energy levels are separated from each other by a greater amount than low energy levels are. The lowest possible energy for the particle (its zero-point energy) is found in state 1, which is given by[9]
The particle, therefore, always has a positive energy. This contrasts with classical systems, where the particle can have zero energy by resting motionlessly. This can be explained in terms of the uncertainty principle, which states that the product of the uncertainties in the position and momentum of a particle is limited by
It can be shown that the uncertainty in the position of the particle is proportional to the width of the box.[10] Thus, the uncertainty in momentum is roughly inversely proportional to the width of the box.[9] The kinetic energy of a particle is given by 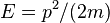 , and hence the minimum kinetic energy of the particle in a box is inversely proportional to the mass and the square of the well width, in qualitative agreement with the calculation above.[9]
, and hence the minimum kinetic energy of the particle in a box is inversely proportional to the mass and the square of the well width, in qualitative agreement with the calculation above.[9]
Higher-dimensional boxes

If a particle is trapped in a two-dimensional box, it may freely move in the  and
and 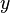 -directions, between barriers separated by lengths
-directions, between barriers separated by lengths 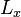 and
and 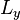 respectively. For a centered box, the position wave function may be written including the length of the box as
respectively. For a centered box, the position wave function may be written including the length of the box as  . Using a similar approach to that of the one-dimensional box, it can be shown that the wavefunctions and energies for a centered box are given respectively by
. Using a similar approach to that of the one-dimensional box, it can be shown that the wavefunctions and energies for a centered box are given respectively by
 ,
,
 ,
,
where the two-dimensional wavevector is given by
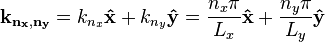 .
.
For a three dimensional box, the solutions are
 ,
,
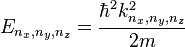 ,
,
where the three-dimensional wavevector is given by:
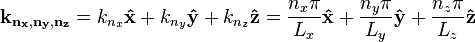 .
.
In general for an n-dimensional box, the solutions are
The 1-dimensional momentum wave functions may likewise be represented by  and the momentum wave function for an n-dimensional centered box is then:
and the momentum wave function for an n-dimensional centered box is then:
An interesting feature of the above solutions is that when two or more of the lengths are the same (e.g.  ), there are multiple wavefunctions corresponding to the same total energy. For example, the wavefunction with
), there are multiple wavefunctions corresponding to the same total energy. For example, the wavefunction with  has the same energy as the wavefunction with
has the same energy as the wavefunction with 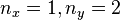 . This situation is called degeneracy and for the case where exactly two degenerate wavefunctions have the same energy that energy level is said to be doubly degenerate. Degeneracy results from symmetry in the system. For the above case two of the lengths are equal so the system is symmetric with respect to a 90° rotation.
. This situation is called degeneracy and for the case where exactly two degenerate wavefunctions have the same energy that energy level is said to be doubly degenerate. Degeneracy results from symmetry in the system. For the above case two of the lengths are equal so the system is symmetric with respect to a 90° rotation.
Applications
Because of its mathematical simplicity, the particle in a box model is used to find approximate solutions for more complex physical systems in which a particle is trapped in a narrow region of low electric potential between two high potential barriers. These quantum well systems are particularly important in optoelectronics, and are used in devices such as the quantum well laser, the quantum well infrared photodetector and the quantum-confined Stark effect modulator. It is also used to model a lattice in the Kronig-Penny model and for a finite metal with the free electron approximation.
Relativistic Effects
The probability density does not go to zero at the nodes if relativistic effects are taken into account.[11]
See also
- History of Quantum Mechanics
- Finite potential well
- Delta function potential
- Gas in a box
- Particle in a ring
- Particle in a spherically symmetric potential
- Quantum harmonic oscillator
- Semicircle potential well
- Configuration integral (statistical mechanics)
- Configuration integral (statistical mechanics), 2008. this wiki site is down; see this article in the web archive on 2012 April 28.
References
- 1 2 3 4 5 Davies, p.4
- ↑ Actually, any constant, finite potential
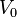 can be specified within the box. This merely shifts the energies of the states by
can be specified within the box. This merely shifts the energies of the states by 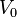 .
. - ↑ Davies, p. 1
- 1 2 Bransden and Joachain, p. 157
- 1 2 Davies p. 5
- 1 2 Bransden and Joachain, p.158
- 1 2 Majernik, Vladimir; Richterek, Lukas (1997-12-01). "Entropic uncertainty relations for the infinite well". J. Phys A 30 (4). doi:10.1088/0305-4470/30/4/002. Retrieved 11 February 2016.
- ↑ Majernik, Vladimir; Majernikova, Eva (1998-12-01). "The momentum entropy of the infinite potential well". J. Phys A 32 (11). doi:10.1088/0305-4470/32/11/013. Retrieved 11 February 2016.
- 1 2 3 Bransden and Joachain, p. 159
- ↑ Davies, p. 15
- ↑ Alberto, P; Fiolhais, C; Gil, V M S (1996). "Relativistic particle in a box" (PDF). European Journal of Physics 17: 19–24. Bibcode:1996EJPh...17...19A. doi:10.1088/0143-0807/17/1/004.
Bibliography
- Bransden, B. H.; Joachain, C. J. (2000). Quantum mechanics (2nd ed.). Essex: Pearson Education. ISBN 0-582-35691-1.
- Davies, John H. (2006). The Physics of Low-Dimensional Semiconductors: An Introduction (6th reprint ed.). Cambridge University Press. ISBN 0-521-48491-X.
- Griffiths, David J. (2004). Introduction to Quantum Mechanics (2nd ed.). Prentice Hall. ISBN 0-13-111892-7.
External links
- Scienceworld (Infinite Potential Well)
- 1-D quantum mechanics java applet simulates particle in a box, as well as other 1-dimensional cases.
- 2-D particle in a box applet