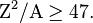Spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 58; spontaneous breakdown into smaller nuclei and a few isolated nuclear particles becomes possible at greater atomic mass numbers.
Because of constraints in forming the daughter fission-product nuclei, spontaneous fission into known nuclides becomes theoretically possible (that is, energetically possible) for some atomic nuclei with atomic masses greater than 92 atomic mass units (amu), with the probability of spontaneous fission increasing as the atomic mass increases above this value.
History
The first nuclear fission process discovered was the fission induced by neutrons. Because cosmic rays produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. The spontaneous fission was identified in 1940 by Soviet physicists Georgy Flyorov and Konstantin Petrzhak[1][2] by their observations of uranium in the Moscow Metro Dinamo station, 60 metres (200 ft) underground.[3]
Cluster decay was shown to be a superasymmetric spontaneous fission process.[4]
Feasibility
Elemental
The lightest natural nuclides that are hypothetically subject to spontaneous fission are niobium-93 and molybdenum-94 (elements 41 and 42, respectively). Spontaneous fission has never been observed in the naturally occurring isotopes of these elements, however. In practice, these are stable isotopes.
Spontaneous fission is feasible over practical observation times only for atomic masses of 232 amu or more. These are elements at least as heavy as thorium-232 – which has a half-life somewhat longer than the age of the universe. Thorium-232 is the lightest primordial nuclide that has left evidence of undergoing spontaneous fission in its minerals.
The known elements most susceptible to spontaneous fission are the synthetic high-atomic-number actinide elements with odd atomic numbers: mendelevium and lawrencium, and also some of the transactinide superheavy elements, such as rutherfordium.
For naturally occurring thorium, uranium-235, and uranium-238, spontaneous fission does occur rarely, but in the vast majority of the radioactive decay of these atoms, alpha decay or beta decay occurs instead. Hence, the spontaneous fission of these isotopes is usually negligible, except in using the exact branching ratios when finding the radioactivity of a sample of these elements.
Mathematical
Mathematically, the criterion for whether spontaneous fission can occur in a time short enough to be observed by present methods, is approximately:
where Z is the atomic number and A is the mass number (e.g. 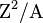 = 36 for uranium-235).
= 36 for uranium-235).
Spontaneous fission rates
Spontaneous fission rates:[6]
| Nuclide | Half-life | Fission prob. per decay | Neutrons per fission | Neutrons per gram-second | Spontaneous half life | Z2/A |
|---|---|---|---|---|---|---|
| 235U | 7.04×108 years | 2.0×10−9 | 1.86 | 3.0×10−4 | 3.5×1017 years | 36.0 |
| 238U | 4.47×109 years | 5.4×10−7 | 2.07 | 0.0136 | 8.4×1015 years | 35.6 |
| 239Pu | 2.41×104 years | 4.4×10−12 | 2.16 | 0.022 | 5.5×1015 years | 37.0 |
| 240Pu | 6569 years | 5.0×10−8 | 2.21 | 920 | 1.16×1011 years | 36.8 |
| 250Cm | 6900 years | 0.61 | 3.31 | 1.6×1010 | N/A | 36.9 |
| 252Cf | 2.638 years | 3.09×10−2 | 3.73 | 2.3×1012 | N/A | 38.1 |
In practice 239Pu will invariably contain a certain amount of 240Pu due to the tendency of 239Pu to absorb an additional neutron during production. 240Pu's high rate of spontaneous fission events makes it an undesirable contaminant. Weapons-grade plutonium contains no more than 7.0% 240Pu.
The rarely used gun-type atomic bomb has a critical insertion time of about one millisecond, and the probability of a fission during this time interval should be small. Therefore only 235U is suitable. Almost all nuclear bombs use some kind of implosion method.
Spontaneous fission can occur much more rapidly when the nucleus of an atom undergoes superdeformation.
Poisson process
Spontaneous fission gives much the same result as induced nuclear fission. However, like other forms of radioactive decay, it occurs due to quantum tunneling, without the atom having been struck by a neutron or other particle as in induced nuclear fission. Spontaneous fissions release neutrons as all fissions do, so if a critical mass is present, a spontaneous fission can initiate a self-sustaining chain reaction. Radioisotopes for which spontaneous fission is not negligible can be used as neutron sources. For example, californium-252 (half-life 2.645 years, SF branch ratio about 3.1 percent) can be used for this purpose. The neutrons released can be used to inspect airline luggage for hidden explosives, to gauge the moisture content of soil in highway and building construction, or to measure the moisture of materials stored in silos, for example.
As long as the spontaneous fission gives a negligible reduction of the number of nuclei that can undergo such fission, this process can be approximated closely as a Poisson process. In this situation, for short time intervals the probability of a spontaneous fission is directly proportional to the length of time.
The spontaneous fission of uranium-238 and uranium-235 does leave trails of damage in the crystal structure of uranium-containing minerals when the fission fragments recoil through them. These trails, or fission tracks, are the foundation of the radiometric dating method called fission track dating.
Notes
- ↑ G. Scharff-Goldhaber and G. S. Klaiber (1946). "Spontaneous Emission of Neutrons from Uranium". Phys. Rev. 70 (3–4): 229–229. Bibcode:1946PhRv...70..229S. doi:10.1103/PhysRev.70.229.2.
- ↑ Igor Sutyagin: The role of nuclear weapons and its possible future missions
- ↑ K. Petrzhak: How the spontaneous fission was discovered (in Russian).
- ↑ Dorin N Poenaru; et al. (1984). "Spontaneous emission of heavy clusters". Journal of Physics G: Nuclear Physics 10: L183–L189. Bibcode:1984JPhG...10L.183P. doi:10.1088/0305-4616/10/8/004.
- ↑ Krane, Kenneth S. (1988). Introductory Nuclear Physics. John Wiley & Sons. pp. 483–484 (Equation 13.3). ISBN 978-0-471-80553-3.
- ↑ Shultis, J. Kenneth; Richard E. Faw (2008). Fundamentals of Nuclear Science and Engineering. CRC Press. pp. 141 (table 6.2). ISBN 1-4200-5135-0.
External links
-
 The LIVEChart of Nuclides - IAEA with filter on spontaneous fission decay
The LIVEChart of Nuclides - IAEA with filter on spontaneous fission decay
| ||||||||||||||||||||