Spermatozoon
| Spermatozoon | |
|---|---|
|
| |
|
Diagram of a human spermatozoon | |
| Details | |
| Identifiers | |
| Latin | spermatozoon |
| MeSH | A05.360.490.890 |
| Dorlands /Elsevier | Spermatozoa |
A spermatozoon (pronounced /ˌspɜːrmætəˈzoʊən/, alternate spelling spermatozoön; plural spermatozoa; from Ancient Greek: σπέρμα "seed" and Ancient Greek: ζῷον "living being") is a motile sperm cell, or moving form of the haploid cell that is the male gamete. A spermatozoon joins an ovum to form a zygote. (A zygote is a single cell, with a complete set of chromosomes, that normally develops into an embryo.)
Sperm cells contribute approximately half of the nuclear genetic information to the diploid offspring (excluding, in most cases, mitochondrial DNA). In mammals, the sex of the offspring is determined by the sperm cell: a spermatozoon bearing a Y chromosome will lead to a male (XY) offspring, while one bearing an X chromosome will lead to a female (XX) offspring. Sperm cells were first observed by Anton van Leeuwenhoek in 1677.[1]
Mammalian spermatozoon structure, function, and size
Humans
The human sperm cell is the reproductive cell in males and will only survive in warm environments; once it leaves the male body the sperm's survival likelihood is reduced and it may die, thereby decreasing the total sperm quality. Sperm cells come in two types, "female" and "male". Sperm cells that give rise to female (XX) offspring after fertilization differ in that they carry an X-chromosome, while sperm cells that give rise to male (XY) offspring carry a Y-chromosome.
Human sperm cells consist of a flat, disc shaped head 5.1 µm by 3.1 µm and a tail 50 µm long.[2] The tail flagellates, which propels the sperm cell (at about 1–3 mm/minute in humans) by whipping in an elliptical cone.[3] Semen has an alkaline nature, and they do not reach full motility (hypermotility) until they reach the vagina where the alkaline pH is neutralized by acidic vaginal fluids. This gradual process takes 20–30 minutes. In this time, fibrinogen from the seminal vesicles forms a clot, securing and protecting the sperm. Just as they become hypermotile, fibrinolysin from the prostate dissolves the clot, allowing the sperm to progress optimally.
The spermatozoon is characterized by a minimum of cytoplasm and the most densely packed DNA known in eukaryotes. Compared to mitotic chromosomes in somatic cells, sperm DNA is at least sixfold more highly condensed.[4]
The specimen contributes with DNA/chromatin, a centriole and perhaps also an oocyte-activating factor (OAF).[5] It may also contribute with paternal messenger RNA (mRNA), also contributing to embryonic development.[5]
-

Electron micrograph of human spermatozoa magnified 3140 times.
-
_-_Spermler_(idrar)_-_01.png)
Sperm cells in the urine sample of a 45-year-old male patient who is being followed with the diagnosis of benign prostate hyperplasia.
-
_-_Spermler_(idrar)_-_02.png)
Another image from the same urine sample as with the image on the left.
The human spermatozoon contains over 6000 different proteins.[6]
DNA damage and repair
DNA damages present in spermatozoa in the period after meiosis but before fertilization may be repaired in the fertilized egg, but if not repaired, can have serious deleterious effects on fertility and the developing embryo. Human spermatozoa are particularly vulnerable to free radical attack and the generation of oxidative DNA damage.[7][8] (see e.g. 8-Oxo-2'-deoxyguanosine)
Exposure of males to certain lifestyle, environmental and/or occupational hazards may increase the risk of aneuploid spermatozoa.[9] In particular, risk of aneuploidy is increased by tobacco smoking,[10][11] and occupational exposure to benzene,[12] insecticides,[13][14] and perfluorinated compounds.[15] Increased aneuploidy of spermatozoa often occurs in association with increased DNA damage.
Avoidance of immune system response
Glycoprotein molecules on the surface of ejaculated sperm cells are recognized by all human female immune systems, and interpreted as a signal that the cell should not be rejected. The female immune system might otherwise attack sperm in the reproductive tract. The specific glycoproteins coating sperm cells are also utilized by some cancerous and bacterial cells, some parasitic worms, and HIV-infected white blood cells, thereby avoiding an immune response from the host organism.[16]
The blood-testis barrier, maintained by the tight junctions between the Sertoli cells of the seminiferous tubules, prevents communication between the forming spermatozoa in the testis and the blood vessels (and immune cells circulating within them) within the interstitial space. This prevents them from eliciting an immune response. The blood-testis barrier is also important in preventing toxic substances from disrupting spermatogenesis.
Spermatozoa in other organisms
Animals
Fertilization relies on spermatozoa for most sexually reproductive animals.
Some species of fruit fly produce the largest known spermatozoon found in nature.[17][18] Drosophila melanogaster produces sperm that can be up to 1.8 mm,[19] while its relative Drosophila bifurca produces the largest known spermatozoon, measuring over 58 mm in size.[17] In D. melanogaster the entire sperm, tail included, gets incorporated into the oocyte cytoplasm, however, for D. bifurca only a small portion of the tail enters the oocyte.[20]
The wood mouse Apodemus sylvaticus possesses spermatozoa with falciform morphology. What makes these gametocytes even more unique is the presence of an apical hook on the sperm head. This hook is used to attach to the hooks or to the flagella of other spermatozoa. Aggregation is caused by these attachments and mobile trains result. These trains provide improved motility in the female reproductive tract and are a means by which fertilization is promoted.[21]
The postmeiotic phase of mouse spermatogenesis is very sensitive to environmental genotoxic agents, because as male germ cells form mature spermatozoa they progressively lose the ability to repair DNA damage.[22] Irradiation of male mice during late spermatogenesis can induce damage that persists for at least 7 days in the fertilizing spermatozoa, and disruption of maternal DNA double-strand break repair pathways increases spermatozoa-derived chromosomal aberrations.[23] Treatment of male mice with melphalan, a bifunctional alkylating agent frequently employed in chemotherapy, induces DNA lesions during meiosis that may persist in an unrepaired state as germ cells progress though DNA repair-competent phases of spermatogenic development.[24] Such unrepaired DNA damages in spermatozoa, after fertilization, can lead to offspring with various abnormalities.
Sea urchins such as Arbacia punctulata—are ideal organisms to use in sperm research, they spawn large numbers of sperm into the sea, making them well-suited as model organisms for experiments.
Plants, algae and fungi
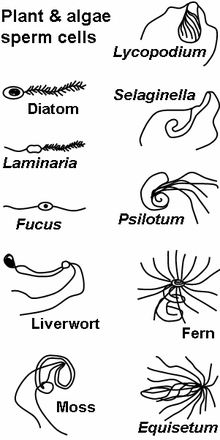
The gametophytes of bryophytes, ferns and some gymnosperms produce motile sperm cells, contrary to pollen grains employed in most gymnosperms and all angiosperms. This renders sexual reproduction in the absence of water impossible, since water is a necessary medium for sperm and egg to meet. Algae and lower plant sperm cells are often multi-flagellated (see image) and thus morphologically different from animal spermatozoa.
Some algae and fungi produce non-motile sperm cells, called spermatia. In higher plants and some algae and fungi, fertilization involves the migration of the sperm nucleus through a fertilization tube (e.g. pollen tube in higher plants) to reach the egg cell.
Spermatozoa production in mammals
Spermatozoa are produced in the seminiferous tubules of the testes in a process called spermatogenesis. Round cells called spermatogonia divide and differentiate eventually to become spermatozoa. During copulation the cloaca or vagina gets inseminated, and then the spermatozoa move through chemotaxis to the ovum inside a Fallopian tube or the uterus.
Spermatozoa activation

Approaching the egg cell is a rather complex, multistep process of chemotaxis guided by different chemical substances/stimuli on individual levels of phylogeny. One of the most significant, common signaling characters of the event is that a prototype of professional chemotaxis receptors, formyl peptide receptor (60.000 receptor/cell) as well as the activator ability of its ligand formyl Met-Leu-Phe have been demonstrated in the surface membrane even in the case of human sperms.[25] Mammalian sperm cells become even more active when they approach an egg cell in a process called sperm activation. Sperm activation has been shown to be caused by calcium ionophores in vitro, progesterone released by nearby cumulus cells and binding to ZP3 of the zona pellucida. The cumulus cells are embedded in a gel-like substance made primarily of hyaluronic acid, and developed in the ovary with the egg and support it as it grows.
The initial change is called "hyperactivation", which causes a change in spermatozoa motility. They swim faster and their tail movements become more forceful and erratic.
A recent discovery links hyperactivation to a sudden influx of calcium ion into the tails. The whip-like tail (flagellum) of the sperm is studded with ion channels formed by proteins called CatSper. These channels are selective, allowing only calcium ions to pass. The opening of CatSper channels is responsible for the influx of calcium. The sudden rise in calcium levels causes the flagellum to form deeper bends, propelling the sperm more forcefully through the viscous environment. Sperm hyperactivity is necessary for breaking through two physical barriers that protect the egg from fertilization.
The second process in sperm activation is the acrosome reaction. This involves releasing the contents of the acrosome, which disperse, and the exposure of enzymes attached to the inner acrosomal membrane of the sperm. This occurs after the sperm first meets the egg. This lock-and-key type mechanism is species-specific and prevents the sperm and egg of different species from fusing. There is some evidence that this binding is what triggers the acrosome to release the enzymes that allow the sperm to fuse with the egg.
ZP3, one of the proteins that make up the zona pellucida, then binds to a partner molecule on the sperm. Enzymes on the inner acrosomal membrane digest the zona pellucida. After the sperm penetrates the zona pellucida, part of the sperm's cell membrane then fuses with the egg cell's membrane, and the contents of the head diffuse into the egg.
Upon penetration, the oocyte is said to have become activated. It undergoes its secondary meiotic division, and the two haploid nuclei (paternal and maternal) fuse to form a zygote. In order to prevent polyspermy and minimise the possibility of producing a triploid zygote, several changes to the egg's zona pellucida renders them impenetrable shortly after the first sperm enters the egg.
Artificial storage
Spermatozoa can be stored in diluents such has the Illini Variable Temperature (IVT) diluent, which have been reported to be able to preserve high fertility of spermatozoa for over seven days.[26] The IVT diluent is composed of several salts, sugars and antibacterial agents and gassed with CO2.[26]
Semen cryopreservation can be used for far longer storage durations. For human spermatozoa, the longest reported successful storage with this method is 21 years.[27]
History
- In 1677 microbiologist Antonie van Leeuwenhoek discovers spermatozoa.
- In 1841 the Swiss anatomist Albert von Kölliker wrote about spermatozoon in his work Untersuchungen über die Bedeutung der Samenfäden.
See also
References
- ↑ "Timeline: Assisted reproduction and birth control". CBC News. Retrieved 2006-04-06.
- ↑ Smith, D.J. (2009). "Human sperm accumulation near surfaces: a simulation study" (PDF). Journal of Fluid Mechanics 621: 295. doi:10.1017/S0022112008004953. Retrieved 20 May 2012.
- ↑ Ishijima, Sumio; Oshio, Shigeru; Mohri, Hideo (1986). "Flagellar movement of human spermatozoa". Gamete research 13 (3): 185–197. doi:10.1002/mrd.1120130302.
- ↑ Ward WS, Coffey DS (1991). "DNA packaging and organization in mammalian spermatozoa: comparison with somatic cells". Biol. Reprod. 44 (4): 569–74. doi:10.1095/biolreprod44.4.569. PMID 2043729.
- 1 2 Developmental sperm contributions: fertilization and beyond Gerardo Barroso, M.D., M.Sc.a, Carlos Valdespin, M.D.a, Eva Vega, M.Sc.a, Ruben Kershenovich, M.D.a, Rosaura Avila, B.Sc.a, Conrado Avendaño, M.D.b, Sergio Oehninger, M.D., Ph.D.b. FertStert, Volume 92, Issue 3, Pages 835-848 (September 2009)
- ↑ Amaral, A.; Castillo, J.; Ramalho-Santos, J.; Oliva, R. (2013). "The combined human sperm proteome: Cellular pathways and implications for basic and clinical science". Human Reproduction Update 20: 40–62. doi:10.1093/humupd/dmt046.
- ↑ Gavriliouk D, Aitken RJ (2015). "Damage to Sperm DNA Mediated by Reactive Oxygen Species: Its Impact on Human Reproduction and the Health Trajectory of Offspring". Adv. Exp. Med. Biol. 868: 23–47. doi:10.1007/978-3-319-18881-2_2. PMID 26178844.
- ↑ Lozano G.M., Bejarano, I., Espino, J., González, D., Ortiz, A., García, J.F., Rodríguez, A.B., Pariente, J.A. (2009). "Relationship between Caspase Activity and Apoptotic Markers in Human Sperm in Response to Hydrogem Peroxide and Progesterone". Journal of Reproduction and Development 55(6): 615-621.
- ↑ Templado C, Uroz L, Estop A (2013). "New insights on the origin and relevance of aneuploidy in human spermatozoa". Mol. Hum. Reprod. 19 (10): 634–43. doi:10.1093/molehr/gat039. PMID 23720770.
- ↑ Shi Q, Ko E, Barclay L, Hoang T, Rademaker A, Martin R (2001). "Cigarette smoking and aneuploidy in human sperm". Mol. Reprod. Dev. 59 (4): 417–21. doi:10.1002/mrd.1048. PMID 11468778.
- ↑ Rubes J, Lowe X, Moore D, Perreault S, Slott V, Evenson D, Selevan SG, Wyrobek AJ (1998). "Smoking cigarettes is associated with increased sperm disomy in teenage men". Fertil. Steril. 70 (4): 715–23. PMID 9797104.
- ↑ Xing C, Marchetti F, Li G, Weldon RH, Kurtovich E, Young S, Schmid TE, Zhang L, Rappaport S, Waidyanatha S, Wyrobek AJ, Eskenazi B (2010). "Benzene exposure near the U.S. permissible limit is associated with sperm aneuploidy". Environ. Health Perspect. 118 (6): 833–9. doi:10.1289/ehp.0901531. PMC 2898861. PMID 20418200.
- ↑ Xia Y, Bian Q, Xu L, Cheng S, Song L, Liu J, Wu W, Wang S, Wang X (2004). "Genotoxic effects on human spermatozoa among pesticide factory workers exposed to fenvalerate". Toxicology 203 (1-3): 49–60. doi:10.1016/j.tox.2004.05.018. PMID 15363581.
- ↑ Xia Y, Cheng S, Bian Q, Xu L, Collins MD, Chang HC, Song L, Liu J, Wang S, Wang X (2005). "Genotoxic effects on spermatozoa of carbaryl-exposed workers". Toxicol. Sci. 85 (1): 615–23. doi:10.1093/toxsci/kfi066. PMID 15615886.
- ↑ Governini L, Guerranti C, De Leo V, Boschi L, Luddi A, Gori M, Orvieto R, Piomboni P (2014). "Chromosomal aneuploidies and DNA fragmentation of human spermatozoa from patients exposed to perfluorinated compounds". Andrologia 47: 1012–9. doi:10.1111/and.12371. PMID 25382683.
- ↑ "Sperm clue to 'disease immunity'". BBC News. 2007-12-17.
- 1 2 Pitnick, S; Spicer, GS; Markow, TA (11 May 1995). "How long is a giant sperm?". Nature 375 (6527): 109. doi:10.1038/375109a0. PMID 7753164.
- ↑ Pitnick, S; Markow, TA (27 September 1994). "Large-male advantages associated with costs of sperm production in Drosophila hydei, a species with giant sperm.". Proceedings of the National Academy of Sciences of the United States of America 91 (20): 9277–81. doi:10.1073/pnas.91.20.9277. PMID 7937755.
- ↑ Cooper, K.W. (1950). Demerec, M., ed. Biology of Drosophila. New York: Wiley. pp. 1–61.
- ↑ Pitnick, S.; Spicer, G. S.; Markow, T. A. (1995). "How long is a giant sperm". Nature 375 (6527): 109. doi:10.1038/375109a0. PMID 7753164.
- ↑ Moore, H; Dvoráková, K; Jenkins, N; Breed, W (2002). ", Exceptional sperm cooperation in Wood Mouse". Nature 418: 174–177. doi:10.1038/nature00832. PMID 12110888.
- ↑ Marchetti F, Wyrobek AJ (2008). "DNA repair decline during mouse spermiogenesis results in the accumulation of heritable DNA damage". DNA Repair (Amst.) 7 (4): 572–81. doi:10.1016/j.dnarep.2007.12.011. PMID 18282746.
- ↑ Marchetti F, Essers J, Kanaar R, Wyrobek AJ (2007). "Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations". Proc. Natl. Acad. Sci. U.S.A. 104 (45): 17725–9. doi:10.1073/pnas.0705257104. PMC 2077046. PMID 17978187.
- ↑ Marchetti F, Bishop J, Gingerich J, Wyrobek AJ (2015). "Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair". Sci Rep 5: 7689. doi:10.1038/srep07689. PMC 4286742. PMID 25567288.
- ↑ Gnessi L, Fabbri A, Silvestroni L, Moretti C, Fraioli F, Pert CB, Isidori A. (1986). "Evidence for the presence of specific receptors for N-formyl chemotactic peptides on human spermatozoa.". J Clin Endocrinol Metab 63 (4): 841–6. doi:10.1210/jcem-63-4-841. PMID 3018025.
- 1 2 Watson, P. F. (1993). "The potential impact of sperm encapsulation technology on the importance of timing of artificial insemination: A perspective in the light of published work". Reproduction, Fertility and Development 5 (6): 691–9. doi:10.1071/RD9930691. PMID 9627729.
- ↑ Planer NEWS and Press Releases > Child born after 21 year semen storage using Planer controlled rate freezer 14/10/2004
External links
| Wikimedia Commons has media related to Spermatozoon diagrams. |
| ||||||||||||||||||||||||||||||||||||
|

