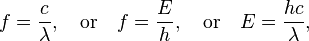Electromagnetic spectrum
| Class | Freq- uency |
Wave- length |
Energy | |||
|---|---|---|---|---|---|---|
| Ionizing radiation |
γ | Gamma rays | 300 EHz | 1 pm | 1.24 MeV | |
| 30 EHz | 10 pm | 124 keV | ||||
| HX | Hard X-rays | |||||
| 3 EHz | 100 pm | 12.4 keV | ||||
| SX | Soft X-rays | |||||
| 300 PHz | 1 nm | 1.24 keV | ||||
| 30 PHz | 10 nm | 124 eV | ||||
| EUV | Extreme ultraviolet |
|||||
| 3 PHz | 100 nm | 12.4 eV | ||||
| NUV | Near ultraviolet |
|||||
| Visible | 300 THz | 1 μm | 1.24 eV | |||
| NIR | Near infrared | |||||
| 30 THz | 10 μm | 124 meV | ||||
| MIR | Mid infrared | |||||
| 3 THz | 100 μm | 12.4 meV | ||||
| FIR | Far infrared | |||||
| 300 GHz | 1 mm | 1.24 meV | ||||
| Micro- waves and radio waves |
EHF | Extremely high frequency |
||||
| 30 GHz | 1 cm | 124 μeV | ||||
| SHF | Super high frequency |
|||||
| 3 GHz | 1 dm | 12.4 μeV | ||||
| UHF | Ultra high frequency |
|||||
| 300 MHz | 1 m | 1.24 μeV | ||||
| VHF | Very high frequency |
|||||
| 30 MHz | 10 m | 124 neV | ||||
| HF | High frequency |
|||||
| 3 MHz | 100 m | 12.4 neV | ||||
| MF | Medium frequency |
|||||
| 300 kHz | 1 km | 1.24 neV | ||||
| LF | Low frequency |
|||||
| 30 kHz | 10 km | 124 peV | ||||
| VLF | Very low frequency |
|||||
| 3 kHz | 100 km | 12.4 peV | ||||
| ULF | Ultra low frequency | |||||
| 300 Hz | 1 Mm | 1.24 peV | ||||
| SLF | Super low frequency |
|||||
| 30 Hz | 10 Mm | 124 feV | ||||
| ELF | Extremely low frequency |
|||||
| 3 Hz | 100 Mm | 12.4 feV | ||||
| Sources: File:Light spectrum.svg [1][2][3] | ||||||
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation.[4] The "electromagnetic spectrum" of an object has a different meaning, and is instead the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object.
The electromagnetic spectrum extends from below the low frequencies used for modern radio communication to gamma radiation at the short-wavelength (high-frequency) end, thereby covering wavelengths from thousands of kilometers down to a fraction of the size of an atom. The limit for long wavelengths is the size of the universe itself, while it is thought that the short wavelength limit is in the vicinity of the Planck length.[5] Until the middle of the 20th century it was believed by most physicists that this spectrum was infinite and continuous.
Most parts of the electromagnetic spectrum are used in science for spectroscopic and other probing interactions, as ways to study and characterize matter.[6] In addition, radiation from various parts of the spectrum has found many other uses for communications and manufacturing (see electromagnetic radiation for more applications).
History of electromagnetic spectrum discovery
For most of history, visible light was the only known part of the electromagnetic spectrum. The ancient Greeks recognized that light traveled in straight lines and studied some of its properties, including reflection and refraction. The study of light continued, and during the 16th and 17th centuries conflicting theories regarded light as either a wave or a particle.
The first discovery of electromagnetic radiation other than visible light came in 1800, when William Herschel discovered infrared radiation.[7] He was studying the temperature of different colors by moving a thermometer through light split by a prism. He noticed that the highest temperature was beyond red. He theorized that this temperature change was due to "calorific rays" that were a type of light ray that could not be seen.
The next year, Johann Ritter, working at the other end of the spectrum, noticed what he called "chemical rays" (invisible light rays that induced certain chemical reactions). These behaved similarly to visible violet light rays, but were beyond them in the spectrum.[8] They were later renamed ultraviolet radiation.
Electromagnetic radiation had been first linked to electromagnetism in 1845, when Michael Faraday noticed that the polarization of light traveling through a transparent material responded to a magnetic field (see Faraday effect). During the 1860s James Maxwell developed four partial differential equations for the electromagnetic field. Two of these equations predicted the possibility of, and behavior of, waves in the field. Analyzing the speed of these theoretical waves, Maxwell realized that they must travel at a speed that was about the known speed of light. This startling coincidence in value led Maxwell to make the inference that light itself is a type of electromagnetic wave.
Maxwell's equations predicted an infinite number of frequencies of electromagnetic waves, all traveling at the speed of light. This was the first indication of the existence of the entire electromagnetic spectrum.
Maxwell's predicted waves included waves at very low frequencies compared to infrared, which in theory might be created by oscillating charges in an ordinary electrical circuit of a certain type. Attempting to prove Maxwell's equations and detect such low frequency electromagnetic radiation, in 1886 the physicist Heinrich Hertz built an apparatus to generate and detect what are now called radio waves. Hertz found the waves and was able to infer (by measuring their wavelength and multiplying it by their frequency) that they traveled at the speed of light. Hertz also demonstrated that the new radiation could be both reflected and refracted by various dielectric media, in the same manner as light. For example, Hertz was able to focus the waves using a lens made of tree resin. In a later experiment, Hertz similarly produced and measured the properties of microwaves. These new types of waves paved the way for inventions such as the wireless telegraph and the radio.
In 1895 Wilhelm Röntgen noticed a new type of radiation emitted during an experiment with an evacuated tube subjected to a high voltage. He called these radiations x-rays and found that they were able to travel through parts of the human body but were reflected or stopped by denser matter such as bones. Before long, many uses were found for them in the field of medicine.
The last portion of the electromagnetic spectrum was filled in with the discovery of gamma rays. In 1900 Paul Villard was studying the radioactive emissions of radium when he identified a new type of radiation that he first thought consisted of particles similar to known alpha and beta particles, but with the power of being far more penetrating than either. However, in 1910, British physicist William Henry Bragg demonstrated that gamma rays are electromagnetic radiation, not particles, and in 1914, Ernest Rutherford (who had named them gamma rays in 1903 when he realized that they were fundamentally different from charged alpha and beta particles) and Edward Andrade measured their wavelengths, and found that gamma rays were similar to X-rays, but with shorter wavelengths and higher frequencies.
Range of the spectrum
Electromagnetic waves are typically described by any of the following three physical properties: the frequency f, wavelength λ, or photon energy E. Frequencies observed in astronomy range from 2.4×1023 Hz (1 GeV gamma rays) down to the local plasma frequency of the ionized interstellar medium (~1 kHz). Wavelength is inversely proportional to the wave frequency,[6] so gamma rays have very short wavelengths that are fractions of the size of atoms, where as wavelengths on the opposite end of the spectrum can be as long as the universe. Photon energy is directly proportional to the wave frequency, so gamma ray photons have the highest energy (around a billion electron volts), while radio wave photons have very low energy (around a femtoelectronvolt). These relations are illustrated by the following equations:
where:
- c = 299792458 m/s is the speed of light in a vacuum
- h = 6.62606896(33)×10−34 J·s = 4.13566733(10)×10−15 eV·s is Planck's constant.[9]
Whenever electromagnetic waves exist in a medium with matter, their wavelength is decreased. Wavelengths of electromagnetic radiation, no matter what medium they are traveling through, are usually quoted in terms of the vacuum wavelength, although this is not always explicitly stated.
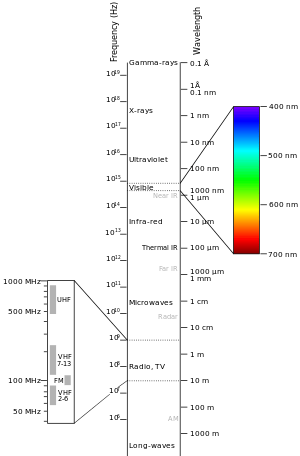
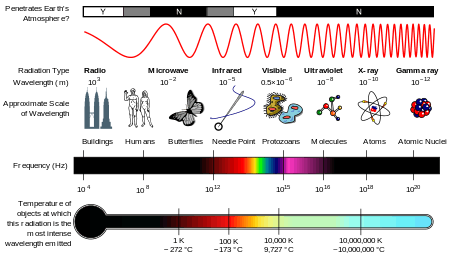
Generally, electromagnetic radiation is classified by wavelength into radio wave, microwave, terahertz (or sub-millimeter) radiation, infrared, the visible region is perceived as light, ultraviolet, X-rays and gamma rays. The behavior of EM radiation depends on its wavelength. When EM radiation interacts with single atoms and molecules, its behavior also depends on the amount of energy per quantum (photon) it carries.
Spectroscopy can detect a much wider region of the EM spectrum than the visible range of 400 nm to 700 nm. A common laboratory spectroscope can detect wavelengths from 2 nm to 2500 nm. Detailed information about the physical properties of objects, gases, or even stars can be obtained from this type of device. Spectroscopes are widely used in astrophysics. For example, many hydrogen atoms emit a radio wave photon that has a wavelength of 21.12 cm. Also, frequencies of 30 Hz and below can be produced by and are important in the study of certain stellar nebulae[10] and frequencies as high as 2.9×1027 Hz have been detected from astrophysical sources.[11]
Rationale for spectrum regional names
Electromagnetic radiation interacts with matter in different ways across the spectrum. These types of interaction are so different that historically different names have been applied to different parts of the spectrum, as though these were different types of radiation. Thus, although these "different kinds" of electromagnetic radiation form a quantitatively continuous spectrum of frequencies and wavelengths, the spectrum remains divided for practical reasons related to these qualitative interaction differences.
| Region of the spectrum | Main interactions with matter |
|---|---|
| Radio | Collective oscillation of charge carriers in bulk material (plasma oscillation). An example would be the oscillatory travels of the electrons in an antenna. |
| Microwave through far infrared | Plasma oscillation, molecular rotation |
| Near infrared | Molecular vibration, plasma oscillation (in metals only) |
| Visible | Molecular electron excitation (including pigment molecules found in the human retina), plasma oscillations (in metals only) |
| Ultraviolet | Excitation of molecular and atomic valence electrons, including ejection of the electrons (photoelectric effect) |
| X-rays | Excitation and ejection of core atomic electrons, Compton scattering (for low atomic numbers) |
| Gamma rays | Energetic ejection of core electrons in heavy elements, Compton scattering (for all atomic numbers), excitation of atomic nuclei, including dissociation of nuclei |
| High-energy gamma rays | Creation of particle-antiparticle pairs. At very high energies a single photon can create a shower of high-energy particles and antiparticles upon interaction with matter. |
Types of radiation
Boundaries
A discussion of the regions (or bands or types) of the electromagnetic spectrum is given below. Note that there are no precisely defined boundaries between the bands of the electromagnetic spectrum; rather they fade into each other like the bands in a rainbow (which is the sub-spectrum of visible light). Radiation of each frequency and wavelength (or in each band) has a mix of properties of the two regions of the spectrum that bound it. For example, red light resembles infrared radiation in that it can excite and add energy to some chemical bonds and indeed must do so to power the chemical mechanisms responsible for photosynthesis and the working of the visual system.
Regions of the spectrum
The types of electromagnetic radiation are broadly classified into the following classes:[6]
- Gamma radiation
- X-ray radiation
- Ultraviolet radiation
- Visible radiation
- Infrared radiation
- Terahertz radiation
- Microwave radiation
- Radio waves
This classification goes in the increasing order of wavelength, which is characteristic of the type of radiation.[6] While, in general, the classification scheme is accurate, in reality there is often some overlap between neighboring types of electromagnetic energy. For example, SLF radio waves at 60 Hz may be received and studied by astronomers, or may be ducted along wires as electric power, although the latter is, in the strict sense, not electromagnetic radiation at all (see near and far field).
The distinction between X-rays and gamma rays is partly based on sources: the photons generated from nuclear decay or other nuclear and subnuclear/particle process, are always termed gamma rays, whereas X-rays are generated by electronic transitions involving highly energetic inner atomic electrons.[12][13][14] In general, nuclear transitions are much more energetic than electronic transitions, so gamma-rays are more energetic than X-rays, but exceptions exist. By analogy to electronic transitions, muonic atom transitions are also said to produce X-rays, even though their energy may exceed 6 megaelectronvolts (0.96 pJ),[15] whereas there are many (77 known to be less than 10 keV (1.6 fJ)) low-energy nuclear transitions (e.g., the 7.6 eV (1.22 aJ) nuclear transition of thorium-229), and, despite being one million-fold less energetic than some muonic X-rays, the emitted photons are still called gamma rays due to their nuclear origin.[16]
The convention that EM radiation that is known to come from the nucleus, is always called "gamma ray" radiation is the only convention that is universally respected, however. Many astronomical gamma ray sources (such as gamma ray bursts) are known to be too energetic (in both intensity and wavelength) to be of nuclear origin. Quite often, in high energy physics and in medical radiotherapy, very high energy EMR (in the >10 MeV region)—which is of higher energy than any nuclear gamma ray—is not called X-ray or gamma-ray, but instead by the generic term of "high energy photons."
The region of the spectrum where a particular observed electromagnetic radiation falls, is reference frame-dependent (due to the Doppler shift for light), so EM radiation that one observer would say is in one region of the spectrum could appear to an observer moving at a substantial fraction of the speed of light with respect to the first to be in another part of the spectrum. For example, consider the cosmic microwave background. It was produced, when matter and radiation decoupled, by the de-excitation of hydrogen atoms to the ground state. These photons were from Lyman series transitions, putting them in the ultraviolet (UV) part of the electromagnetic spectrum. Now this radiation has undergone enough cosmological red shift to put it into the microwave region of the spectrum for observers moving slowly (compared to the speed of light) with respect to the cosmos.
Radio frequency
Radio waves generally are utilized by antennas of appropriate size (according to the principle of resonance), with wavelengths ranging from hundreds of meters to about one millimeter. They are used for transmission of data, via modulation. Television, mobile phones, wireless networking, and amateur radio all use radio waves. The use of the radio spectrum is regulated by many governments through frequency allocation.
Radio waves can be made to carry information by varying a combination of the amplitude, frequency, and phase of the wave within a frequency band. When EM radiation impinges upon a conductor, it couples to the conductor, travels along it, and induces an electric current on the surface of that conductor by exciting the electrons of the conducting material. This effect (the skin effect) is used in antennas.
Microwaves

The super-high frequency (SHF) and extremely high frequency (EHF) of microwaves are on the short side of radio waves. Microwaves are waves that are typically short enough (measured in millimeters) to employ tubular metal waveguides of reasonable diameter. Microwave energy is produced with klystron and magnetron tubes, and with solid state diodes such as Gunn and IMPATT devices. Microwaves are absorbed by molecules that have a dipole moment in liquids. In a microwave oven, this effect is used to heat food. Low-intensity microwave radiation is used in Wi-Fi, although this is at intensity levels unable to cause thermal heating.
Volumetric heating, as used by microwave ovens, transfers energy through the material electromagnetically, not as a thermal heat flux. The benefit of this is a more uniform heating and reduced heating time; microwaves can heat material in less than 1% of the time of conventional heating methods.
When active, the average microwave oven is powerful enough to cause interference at close range with poorly shielded electromagnetic fields such as those found in mobile medical devices and poorly made consumer electronics.
Terahertz radiation
Terahertz radiation is a region of the spectrum between far infrared and microwaves. Until recently, the range was rarely studied and few sources existed for microwave energy at the high end of the band (sub-millimeter waves or so-called terahertz waves), but applications such as imaging and communications are now appearing. Scientists are also looking to apply terahertz technology in the armed forces, where high-frequency waves might be directed at enemy troops to incapacitate their electronic equipment.[17]
Infrared radiation
The infrared part of the electromagnetic spectrum covers the range from roughly 300 GHz to 400 THz (1 mm - 750 nm). It can be divided into three parts:[6]
- Far-infrared, from 300 GHz to 30 THz (1 mm - 10 μm). The lower part of this range may also be called microwaves or terahertz waves. This radiation is typically absorbed by so-called rotational modes in gas-phase molecules, by molecular motions in liquids, and by phonons in solids. The water in Earth's atmosphere absorbs so strongly in this range that it renders the atmosphere in effect opaque. However, there are certain wavelength ranges ("windows") within the opaque range that allow partial transmission, and can be used for astronomy. The wavelength range from approximately 200 μm up to a few mm is often referred to as "sub-millimeter" in astronomy, reserving far infrared for wavelengths below 200 μm.
- Mid-infrared, from 30 to 120 THz (10 - 2.5 μm). Hot objects (black-body radiators) can radiate strongly in this range, and human skin at normal body temperature radiates strongly at the lower end of this region. This radiation is absorbed by molecular vibrations, where the different atoms in a molecule vibrate around their equilibrium positions. This range is sometimes called the fingerprint region, since the mid-infrared absorption spectrum of a compound is very specific for that compound.
- Near-infrared, from 120 to 400 THz (2,500 - 750 nm). Physical processes that are relevant for this range are similar to those for visible light. The highest frequences in this region can be detected directly by some types of photographic film, and by many types of solid state image sensors for infrared photography and videography.
Visible radiation (light)
Above infrared in frequency comes visible light. The Sun emits its peak power in the visible region, although integrating the entire emission power spectrum through all wavelengths shows that the Sun emits slightly more infrared than visible light.[18] By definition, visible light is the part of the EM spectrum the human eye is the most sensitive to. Visible light (and near-infrared light) is typically absorbed and emitted by electrons in molecules and atoms that move from one energy level to another. This action allows the chemical mechanisms that underlie human vision and plant photosynthesis. The light that excites the human visual system is a very small portion of the electromagnetic spectrum. A rainbow shows the optical (visible) part of the electromagnetic spectrum; infrared (if it could be seen) would be located just beyond the red side of the rainbow with ultraviolet appearing just beyond the violet end.
Electromagnetic radiation with a wavelength between 380 nm and 760 nm (400–790 terahertz) is detected by the human eye and perceived as visible light. Other wavelengths, especially near infrared (longer than 760 nm) and ultraviolet (shorter than 380 nm) are also sometimes referred to as light, especially when the visibility to humans is not relevant. White light is a combination of lights of different wavelengths in the visible spectrum. Passing white light through a prism splits it up into the several colors of light observed in the visible spectrum between 400 nm and 780 nm.
If radiation having a frequency in the visible region of the EM spectrum reflects off an object, say, a bowl of fruit, and then strikes the eyes, this results in visual perception of the scene. The brain's visual system processes the multitude of reflected frequencies into different shades and hues, and through this insufficiently-understood psychophysical phenomenon, most people perceive a bowl of fruit.
At most wavelengths, however, the information carried by electromagnetic radiation is not directly detected by human senses. Natural sources produce EM radiation across the spectrum, and technology can also manipulate a broad range of wavelengths. Optical fiber transmits light that, although not necessarily in the visible part of the spectrum (it is usually infrared), can carry information. The modulation is similar to that used with radio waves.
Ultraviolet radiation

Next in frequency comes ultraviolet (UV). The wavelength of UV rays is shorter than the violet end of the visible spectrum but longer than the X-ray.
UV in the very shortest wavelength range (next to X-rays) is capable of ionizing atoms (see photoelectric effect), greatly changing their physical behavior.
At the middle range of UV, UV rays cannot ionize but can break chemical bonds, making molecules unusually reactive. Sunburn, for example, is caused by the disruptive effects of middle range UV radiation on skin cells, which is the main cause of skin cancer. UV rays in the middle range can irreparably damage the complex DNA molecules in the cells producing thymine dimers making it a very potent mutagen.
The Sun emits significant UV radiation (about 10% of its total power), including extremely short wavelength UV that could potentially destroy most life on land (ocean water would provide some protection for life there). However, most of the Sun's damaging UV wavelengths are absorbed by the atmosphere and ozone layer before they reach the surface. The higher energy (shortest wavelength) ranges of UV (called "vacuum UV") are absorbed by nitrogen and, at longer wavelengths, by simple diatomic oxygen in the air. Most of the UV in the mid-range of energy is blocked by the ozone layer, which absorbs strongly in the important 200–315 nm range, the lower energy part of which is too long for ordinary dioxygen in air to absorb. The very lowest energy range of UV between 315 nm and visible light (called UV-A) is not blocked well by the atmosphere, but does not cause sunburn and does less biological damage. However, it is not harmless and does create oxygen radicals, mutations and skin damage. See ultraviolet for more information.
X-rays
After UV come X-rays, which, like the upper ranges of UV are also ionizing. However, due to their higher energies, X-rays can also interact with matter by means of the Compton effect. Hard X-rays have shorter wavelengths than soft X-rays and as they can pass through many substances with little absorption, they can be used to 'see through' objects with 'thicknesses' less than that equivalent to a few meters of water. One notable use is diagnostic X-ray imaging in medicine (a process known as radiography). X-rays are useful as probes in high-energy physics. In astronomy, the accretion disks around neutron stars and black holes emit X-rays, enabling studies of these phenomena. X-rays are also emitted by the coronas of stars and are strongly emitted by some types of nebulae. However, X-ray telescopes must be placed outside the Earth's atmosphere to see astronomical X-rays, since the great depth of the atmosphere of Earth is opaque to X-rays (with areal density of 1000 grams per cm2), equivalent to 10 meters thickness of water.[19] This is an amount sufficient to block almost all astronomical X-rays (and also astronomical gamma rays—see below).
Gamma rays
After hard X-rays come gamma rays, which were discovered by Paul Villard in 1900. These are the most energetic photons, having no defined lower limit to their wavelength. In astronomy they are valuable for studying high-energy objects or regions, however as with X-rays this can only be done with telescopes outside the Earth's atmosphere. Gamma rays are used experimentally by physicists for their penetrating ability and are produced by a number of radioisotopes. They are used for irradiation of foods and seeds for sterilization, and in medicine they are occasionally used in radiation cancer therapy.[20] More commonly, gamma rays are used for diagnostic imaging in nuclear medicine, an example being PET scans. The wavelength of gamma rays can be measured with high accuracy through the effects of Compton scattering.
See also
Notes and references
- ↑ What is Light? – UC Davis lecture slides Archived July 23, 2014 at the Wayback Machine
- ↑ Elert, Glenn. "The Electromagnetic Spectrum, The Physics Hypertextbook". Hypertextbook.com. Retrieved 2010-10-16.
- ↑ "Definition of frequency bands on". Vlf.it. Retrieved 2010-10-16.
- ↑ "Imagine the Universe! Dictionary". NASA. Archived from the original on 4 February 2015.
- ↑ Bakshi, U. A. and Godse, A. P. (2009). Basic Electronics Engineering. Technical Publications. pp. 8–10. ISBN 978-81-8431-580-6.
- 1 2 3 4 5 Mehta, Akul. "Introduction to the Electromagnetic Spectrum and Spectroscopy". Pharmaxchange.info. Retrieved 2011-11-08.
- ↑ "Herschel Discovers Infrared Light". Cool Cosmos Classroom activities. Archived from the original on 2012-02-25. Retrieved 4 March 2013.
He directed sunlight through a glass prism to create a spectrum […] and then measured the temperature of each colour. […] He found that the temperatures of the colors increased from the violet to the red part of the spectrum. […] Herschel decided to measure the temperature just beyond the red of the spectrum in a region where no sunlight was visible. To his surprise, he found that this region had the highest temperature of all.
- ↑ Davidson, Michael W. "Johann Wilhelm Ritter (1776–1810)". The Florida State University. Retrieved 5 March 2013.
Ritter […] hypothesized that there must also be invisible radiation beyond the violet end of the spectrum and commenced experiments to confirm his speculation. He began working with silver chloride, a substance decomposed by light, measuring the speed at which different colours of light broke it down. […] Ritter […] demonstrated that the fastest rate of decomposition occurred with radiation that could not be seen, but that existed in a region beyond the violet. Ritter initially referred to the new type of radiation as chemical rays, but the title of ultraviolet radiation eventually became the preferred term.
- ↑ Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006". Rev. Mod. Phys. 80 (2): 633–730. arXiv:0801.0028. Bibcode:2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633. Direct link to value.
- ↑ Condon, J. J. and Ransom, S. M. "Essential Radio Astronomy: Pulsar Properties". National Radio Astronomy Observatory. Retrieved 2008-01-05.
- ↑ Abdo, A. A.; Allen, B.; Berley, D.; Blaufuss, E.; Casanova, S.; Chen, C.; Coyne, D. G.; Delay, R. S.; Dingus, B. L.; Ellsworth, R. W.; Fleysher, L.; Fleysher, R.; Gebauer, I.; Gonzalez, M. M.; Goodman, J. A.; Hays, E.; Hoffman, C. M.; Kolterman, B. E.; Kelley, L. A.; Lansdell, C. P.; Linnemann, J. T.; McEnery, J. E.; Mincer, A. I.; Moskalenko, I. V.; Nemethy, P.; Noyes, D.; Ryan, J. M.; Samuelson, F. W.; Saz Parkinson, P. M.; et al. (2007). "Discovery of TeV Gamma-Ray Emission from the Cygnus Region of the Galaxy". The Astrophysical Journal 658: L33. arXiv:astro-ph/0611691. Bibcode:2007ApJ...658L..33A. doi:10.1086/513696.
- ↑ Feynman, Richard; Leighton, Robert and Sands, Matthew (1963). The Feynman Lectures on Physics, Vol.1. USA: Addison-Wesley. pp. 2–5. ISBN 0-201-02116-1.
- ↑ L'Annunziata, Michael and Baradei, Mohammad (2003). Handbook of Radioactivity Analysis. Academic Press. p. 58. ISBN 0-12-436603-1.
- ↑ Grupen, Claus; Cowan, G.; Eidelman, S. D. and Stroh, T. (2005). Astroparticle Physics. Springer. p. 109. ISBN 3-540-25312-2.
- ↑ Corrections to muonic X-rays and a possible proton halo slac-pub-0335 (1967)
- ↑ "Gamma-Rays". Hyperphysics.phy-astr.gsu.edu. Retrieved 2010-10-16.
- ↑ "Advanced weapon systems using lethal Short-pulse terahertz radiation from high-intensity-laser-produced plasmas". India Daily. March 6, 2005. Archived from the original on 6 January 2010. Retrieved 2010-09-27.
- ↑ "Reference Solar Spectral Irradiance: Air Mass 1.5". Retrieved 2009-11-12.
- ↑ Koontz, Steve (26 June 2012) Designing Spacecraft and Mission Operations Plans to Meet Flight Crew Radiation Dose. NASA/MIT Workshop. See pages I-7 (atmosphere) and I-23 (for water).
- ↑ Uses of Electromagnetic Waves | gcse-revision, physics, waves, uses-electromagnetic-waves | Revision World
External links
| Wikimedia Commons has media related to Electromagnetic spectrum. |
- UnwantedEmissions.com (U.S. radio spectrum allocations resource)
- Australian Radiofrequency Spectrum Allocations Chart (from Australian Communications and Media Authority)
- Canadian Table of Frequency Allocations (from Industry Canada)
- U.S. Frequency Allocation Chart — Covering the range 3 kHz to 300 GHz (from Department of Commerce)
- UK frequency allocation table (from Ofcom, which inherited the Radiocommunications Agency's duties, pdf format)
- Flash EM Spectrum Presentation / Tool – Very complete and customizable.
- How to render the color spectrum / Code – Only approximately right.
- Poster "Electromagnetic Radiation Spectrum" (992 kB)
- Electromagnetic Spectrum presentation
- Electromagnetic Spectrum Strategy: A Call to Action U.S. Department of Defense
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||