Sodium polysulfide
| Sodium pentasulfide, a representative component of sodium polysulfide | |
 | |
| Names | |
|---|---|
| Other names
Sodium sulfane; Viradon | |
| Identifiers | |
| 1344-08-7 | |
| UN number | UN3266 |
| Properties | |
| Na2Sx | |
| Hazards | |
| Safety data sheet | AGFA |
| NFPA 704 | |
| Flash point | Non-combustible |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2-, called polysulfide anions, include disulfide (S22-), trisulfide (S32-), tetrasulfide (S42-), and pentasulfide (S52-). In principle, but not in practice, the chain lengths could be longer.[1] The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Structure
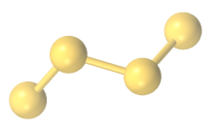
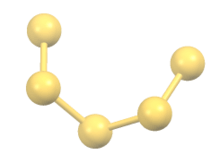
The polysulfide anions form chains with S---S bond distances around 2 Å in length. The chains adopt skewed conformations. In the solid state, these salts are dense solids with strong association of the sodium cations with the anionic termini of the chains.[2]
Production and occurrence
Sodium polysulfide can produced by dissolving sulfur in a solution of sodium sulfide.[3] Alternatively they are produced by the redox reaction of aqueous sodium hydroxide with sulfur at elevated temperatures.[4] Finally they arise by the reduction of elemental sulfur with sodium, a reaction often conducted in anhydrous ammonia.
These salts are used in the production of polysulfide polymers, as a chemical fungicide, as a blackening agent on copper jewellery, as a component in a polysulfide bromide battery, as a toner in a photochemical solution, and in the tanning industry to remove hair from hides.
Reactions
As exploited in the sodium-sulfur battery, the polysulfides absorb and release reducing equivalents by breaking and making S-S bonds, respectively. An idealized reaction for sodium tetrasulfide is shown:
- Na2S4 + 2 Na
 2 Na2S2
2 Na2S2
Alkylation gives organic polysulfides according to the following idealized equation:
- Na2S4 + 2 RX → 2 NaX + R2S4
Alkylation with an organic dihalide gives polymers called thiokols.
Protonation of these salts gives hydrogen sulfide and elemental sulfur, as illustrated by the reaction of sodium pentasulfide:
- Na2S5 + 2 H+ → H2S + 1/2 S8
References
- ↑ Elemental Sulfur and Sulfur-Rich Compounds volumes I and II, Steudel, R., Ed.; Springer-Verlag: Berlin, 2003.
- ↑ Rosén, E.; Tegman, R. "Preparative and X - ray powder diffraction study of the polysulfides Na2S2, Na2S4 and Na2S5" Acta Chemica Scandinavica 1988, volume 25, pp 3329-3336. doi:10.3891/acta.chem.scand.25-3329
- ↑ F. Fehér" Sodium Disulfide", "Sodium Tetrasulfide" "Sodium Pentasulfide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 361-367.
- ↑ Lee, T.C.P. (1999). Properties and applications of elastomeric polysulfides. Rapra Technology. p. 4. ISBN 978-1859571583.
