Eusociality
Eusociality (Greek eu: "good/real" + "social"), the highest level of organization of animal sociality, is defined by the following characteristics: cooperative brood care (including brood care of offspring from other individuals), overlapping generations within a colony of adults, and a division of labor into reproductive and non-reproductive groups. The division of labor creates specialized behavioral groups within an animal society which are sometimes called castes. Eusociality is distinguished from all other social systems because individuals of at least one caste usually lose the ability to perform at least one behavior characteristic of individuals in another caste.
Eusociality exists in certain insects, crustaceans and possibly mammals. It is mostly observed and studied in the Hymenoptera (ants, bees, and wasps) and in the termites. For example, a colony has caste differences; queens and reproductive males take the roles as the sole reproducers while the soldiers and workers work together to create a living situation favorable for the brood. In addition to Hymenoptera and Isoptera, there are two known eusocial vertebrates from the order Rodentia, which includes the naked mole-rat and the Damaraland mole-rat. Some shrimps such as Synalpheus regalis are also eusocial.
Several other levels of animal sociality have been distinguished. These include presocial (solitary but social), subsocial, and parasocial (including communal, quasisocial, and semisocial).
History

The term "eusocial" was introduced in 1966 by Suzanne Batra who used it to describe nesting behavior in Halictine bees.[1][2] Batra observed the cooperative behavior of the bees, males and females alike, as they took responsibility for at least one duty (i.e. burrowing, cell construction, oviposition) within the colony. The cooperativeness was essential as the activity of one labor division greatly influenced the activity of another. For example, the size of pollen balls, a source of food, depended on when the egg-laying females oviposited. If the provisioning by pollen collectors was incomplete by the time the egg-laying female occupied a cell and oviposited, the size of the pollen balls would be small, leading to small offspring.[2] Batra applied this term to species in which a colony is started by a single individual. Batra described other species, where the founder is accompanied by numerous helpers—as in a swarm of bees or ants—as "hypersocial."
In 1969, Charles D. Michener[3] further expanded Batra’s classification with his comparative study of social behavior in bees. He observed multiple species of bees (Apoidea) in order to investigate the different levels of animal sociality, all of which are different stages that a colony may pass through. Eusociality, which is the highest level of animal sociality a species can attain, specifically had three characteristics that distinguished it from the other levels:
- "Egg-layers and worker-like individuals among adult females" (division of labor)
- The overlap of generations (mother and adult offspring)
- Cooperative work on the cells of the bees' honeycomb[1]
E. O. Wilson then extended the terminology to include other social insects; such as ants, wasps, and termites. Originally, it was defined to include organisms (only invertebrates) that had the following three features:[1][4][5][6]
- Reproductive division of labor (with or without sterile castes)
- Overlapping generations
- Cooperative care of young
As eusociality became a recognized widespread phenomenon, however, it was also discovered in Chordata, specifically Rodentia.
Further research also distinguished another possible important criterion for eusociality, known as "the point of no return". This phenomenon is characterized by eusocial individuals that become fixed into one behavioral group, which usually occurs before reproductive maturity. This prevents them from transitioning between behavioral groups and creates an animal society that is truly dependent on each other for survival and reproductive success. For many insects, this irreversibility has changed the anatomy of the worker caste, which is sterile and provides support for the reproductive caste.[1][6]
Examples
Most eusocial societies exist in arthropods, while few are found in mammals.
In insects

The order Hymenoptera contains the largest group of eusocial insects, including ants, bees, and wasps – those with reproductive "queens" and more or less sterile "workers" and/or "soldiers" that perform specialized tasks.[7] Within the Polistes versicolor, dominant females perform tasks such as building new cells and ovipositing, while subordinate females tend to perform tasks like feeding the larvae and foraging. The task differentiation between castes can be seen in the fact that subordinates complete 81.4% of the total foraging activity, while dominants only complete 18.6% of the total foraging.[8] Another example of this is in the species Apoica flavissima. While only a moderate percentage of species in bees (families Apidae and Halictidae) and wasps (Crabronidae and Vespidae) are eusocial, nearly all species of ants (Formicidae) are eusocial. Some major lineages within these groups are mostly or entirely eusocial, as well, such as the bee tribes Apini, Bombini, Euglossini, and Meliponini, and the wasp subfamilies Polistinae and Vespinae. Eusociality in these families is sometimes managed by a set of pheromones that alter the behavior of specific castes in the colony. These pheromones may act across different species, as observed in Apis andreniformis (black dwarf honey bee), where worker bees responded to queen pheromone from the related Apis florea (red dwarf honey bee).[9] Pheromones are sometimes used in these castes to assist with foraging. Workers of the Australian stingless bee Tetragonula carbonaria, for instance, mark food sources with a pheromone, helping their nest mates to find the food.[10] Reproductive specialization generally involves the production of sterile members of the species, which carry out specialized tasks to care for the reproductive members. It can manifest in the appearance of individuals within a group whose behavior or morphology is modified for group defense, including self-sacrificing behavior ("altruism"). An example of a species whose sterile caste displays this altruistic behavior is Myrmecocystus mexicanus, one of the species of honey ant. Select sterile workers fill their abdomens with liquid food until they become immobile and hang from the ceilings of the underground nests, acting as food storage for the rest of the colony.[11] Not all social species of insects have distinct morphological differences between castes. For example, in the Neotropical social wasp Synoeca surinama, social displays determine the caste ranks of individuals in the developing brood.[12] These castes are sometimes further specialized in their behavior based on age. For example,Scaptotrigona postica workers assume different roles in the nest based on their age. Between approximately 0–40 days old, the workers perform tasks within the nest such as provisioning cell broods, colony cleaning, and nectar reception and dehydration. Once older than 40 days, Scaptotrigona postica workers move outside of the nest to practice colony defense and foraging.[13] In Lasioglossum aeneiventre, a halictid bee from Central America, nests may be headed by more than one female; such nests have more cells, and the number of active cells per female is correlated with the number of females in the nest, implying that having more females leads to more efficient building and provisioning of cells.[14] In similar species with only one queen, such as Lasioglossum malachurum in Europe, the degree of eusociality depends on the clime in which the species is found.[15]
Termites (order Blattodea, infraorder Isoptera) make up another large portion of highly advanced eusocial animals. The colony is differentiated into various castes: the queen and king are the sole reproducing individuals; workers forage and maintain food and resources;[16] and soldiers defend the colony against ant attacks. The latter two castes, which are sterile and perform highly specialized, complex social behaviors, are derived from different stages of pluripotent larvae produced by the reproductive caste.[17] Some soldiers have jaws so enlarged (specialized for defense and attack) that they are unable to feed for themselves and must be fed by workers.[18]
Austroplatypus incompertus is a species of ambrosia beetle native to Australia, and is the first beetle (order Coleoptera) to be recognized as eusocial.[19][20] This species forms colonies in which a single female is fertilized and protected by many unfertilized females which also serve as workers excavating tunnels in trees. This species also participates in cooperative brood care, in which individuals care for juveniles that are not their own.[20]
Some species of gall-inducing insects, including the gall-forming aphid, Pemphigus spyrothecae (order Hemiptera), and thrips (order Thysanoptera), were also described as eusocial.[21][22] These species have very high relatedness among individuals due to their partially asexual mode of reproduction (sterile soldier castes being clones of the reproducing female), but the gall-inhabiting behavior gives these species a defensible resource that sets them apart from related species with similar genetics. They produce soldier castes capable of fortress defense and protection of their colony against both predators and competitors. In these groups, therefore, high relatedness alone does not lead to the evolution of social behavior, but requires that groups occur in a restricted, shared area.[23] These species have morphologically distinct soldier castes that defend against kleptoparasites (parasitism by theft) and are able to reproduce parthenogenetically (without fertilization).[24]
In crustaceans
Eusociality has also arisen three different times among some crustaceans that live in separate colonies. Synalpheus regalis, Synalpheus filitigitus, and Synalpheus chacei, three species of parasitic shrimp that rely on fortress defense and live in groups of closely related individuals in tropical reefs and sponges,[25] live eusocially with a single breeding female and a large number of male defenders, armed with enlarged snapping claws. As with other eusocial societies, there is a single shared living space for the colony members, and the non-breeding members act to defend it.[26]
The fortress defense hypothesis additionally points out that because sponges provide both food and shelter, there is an aggregation of relatives (because the shrimp do not have to disperse to find food), and much competition for those nesting sites. Being the target of attack promotes a good defense system (soldier caste); soldiers therefore promote the fitness of the whole nest by ensuring safety and reproduction of the queen.[27]
Eusociality offers a competitive advantage in shrimp populations. Eusocial species were found to be more abundant, occupy more of the habitat, and use more of the available resources than non-eusocial species.[28][29][30] Other studies add to these findings by pointing out that cohabitation was more rare than expected by chance, and that most sponges were dominated by one species, which was frequently eusocial.
In mammals

Among mammals, ‘true’ eusociality is unknown outside the Bathyergidae,[31] though E. O. Wilson argues that humans are eusocial.[32]
Mammalian examples include the naked mole-rat (Heterocephalus glaber) and the Damaraland mole-rat (Fukomys damarensis)[31] two species of vertebrates that are diploid and highly inbred. Usually living in harsh or limiting environments, these mole-rats aid in raising siblings and relatives born to a single reproductive queen. However, this classification is controversial owing to disputed definitions of 'eusociality.' A study conducted by O’Riain and Faulkes in 2008 suggests that due to regular inbreeding avoidance, mole rats sometimes outbreed and establish new colonies when resources are sufficient.[33] Most of the individuals cooperatively care for the brood of a single reproductive female (the queen) to which they are most likely related.[34] Thus, it is uncertain whether mole rats classify as true eusocial organisms, since their social behavior depends largely on their resources and environment.
Some mammals within the Carnivora and Primates exhibit eusocial tendencies. Perhaps most notable are meerkats (Suricata suricatta) and dwarf mongooses (Helogale parvula). These show cooperative breeding and marked reproductive skews. In the dwarf mongoose, the breeding pair receives food priority and protection from subordinates and rarely has to defend against predators.[35]
Evolution
Phylogenetic distribution
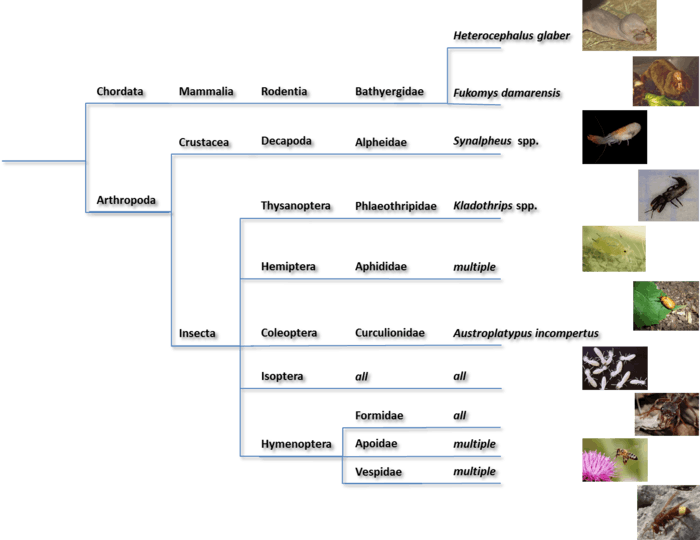
Eusociality is a rare but widespread phenomenon originating in members of the seven aforementioned orders (Isoptera is now an infraorder)- Rodentia (mole-rat)s, Decapoda (snapping shrimp), Thysanoptera (thrips), Hemiptera (aphids), Isoptera (termites), Coleoptera (ambrosia beetles), and Hymenoptera (ants, bees, and wasps). All species of termites are eusocial, and it is believed that they were the first eusocial animals to evolve, sometime in the upper Jurassic period (~150 million years ago).[36] All other orders also contain non-eusocial species, including many lineages where eusociality was inferred to be the ancestral state. Thus the number of independent evolutions of eusociality is still under investigation.
Paradox
Eusociality presents an apparent evolutionary paradox: if adaptive evolution unfolds by differential reproduction of individuals, how can individuals incapable of passing on their genes evolve and persist? In On the Origin of Species, Darwin referred to the existence of sterile castes as the "one special difficulty, which at first appeared to me insuperable, and actually fatal to my theory".[37] Darwin anticipated that a possible resolution to the paradox might lie in the close family relationship, which W.D. Hamilton quantified a century later with his 1964 inclusive fitness theory.
Inclusive fitness and haplodiploidy
According to inclusive fitness theory, organisms can gain fitness not just through increasing their own reproductive output, but also via increasing the reproductive output of other individuals that share their genes, especially their close relatives. Individuals are selected to help their relatives when the cost of helping is less than the benefit gained by their relative multiplied by the fraction of genes that they share, i.e. when Cost < relatedness * Benefit. Under inclusive fitness theory, the necessary conditions for eusociality to evolve are more easily fulfilled by haplodiploid species because of their unusual relatedness structure. In haplodiploid species, females develop from fertilized eggs and males develop from unfertilized eggs. Because a male is haploid, his daughters share 100% of his genes and 50% of their mother's. Therefore, they share 75% of their genes with each other. This mechanism of sex determination gives rise to what W. D. Hamilton first termed "supersisters" which are more related to their sisters than they would be to their own offspring.[38] Even though workers often do not reproduce, they can potentially pass on more of their genes by helping to raise their sisters than they would by having their own offspring (each of which would only have 50% of their genes). This unusual situation, where females may have greater fitness when they help rear siblings rather than producing offspring, is often invoked to explain the multiple independent evolutions of eusociality (arising at least nine separate times) within the haplodiploid group Hymenoptera. However, some eusocial species have been discovered that are not haplodiploid (including termites, some snapping shrimp, and mole rats). Conversely many bees are haplodiploid yet are not eusocial, and among eusocial species many queens mate with multiple males, resulting in a hive of half-sisters that share only 25% of their genes. The association between haplodiploidy and eusociality is below statistical significance.[39] Haplodiploidy alone is thus neither necessary nor sufficient for eusociality to emerge. However relatedness does still play a part, as monogamy (queens mating singly) has been shown to be the ancestral state for all eusocial species so far investigated.[40]
Ecology
Many scientists citing the close phylogenetic relationships between eusocial and non-eusocial species are making the case that environmental factors are especially important in the evolution of eusociality. The relevant factors primarily involve the distribution of food and predators.
With the exception of some aphids, all eusocial species live in a communal nest which provides both shelter and access to food resources. Mole rats and ants live in underground burrows; wasps, bees, and some termites build above-ground hives; thrips and aphids inhabit galls (neoplastic outgrowths) induced on plants; ambrosia beetles and some termites nest together in dead wood; and snapping shrimp inhabit crevices in marine sponges. For many species the habitat outside the nest is often extremely arid or barren, creating such a high cost to dispersal that the chance to take over the colony following parental death is greater than the chance of dispersing to form a new colony. Defense of such fortresses from both predators and competitors often favors the evolution of non-reproductive soldier castes, while the high costs of nest construction and expansion favor non-reproductive worker castes.
The importance of ecology is supported by evidence such as experimentally induced reproductive division of labor, for example when normally solitary queens are forced together.[41] Conversely, female Damaraland mole-rats undergo hormonal changes that promote dispersal after periods of high rainfall,[42] supporting the plasticity of eusocial traits in response to environmental cues.
Multilevel selection
Once pre-adaptations such as group formation, nest building, high cost of dispersal, and morphological variation are present, between-group competition has been cited as a quintessential force in the transition to advanced eusociality. Because the hallmarks of eusociality will produce an extremely altruistic society, such groups will out-reproduce their less cooperative competitors, eventually eliminating all non-eusocial groups from a species.[43]
Multilevel selection has been heavily criticized by some for its conflict with the kin selection theory.[44]
Reversal to solitarity
A reversal to solitarity is an evolutionary phenomenon in which descendants of a eusocial group evolve solitary behavior once again. Bees have been model organisms for the study of reversal to solitarity, because of the diversity of their social systems. Each of the four origins of eusociality in bees was followed by at least one reversal to solitarity, giving a total of at least nine reversals.[3][4] This suggests that eusociality is costly to maintain, and can only persist when ecological variables favor it. Disadvantages of eusociality include the cost of investing in non-reproductive offspring, and an increased risk of disease.[45]
All reversals to solitarity have occurred among primitively eusocial groups; none have followed the emergence of advanced eusociality. The "point of no return" hypothesis posits that the morphological differentiation of reproductive and non-reproductive castes prevents highly eusocial species such as the honeybee from reverting to the solitary state.[46]
Physiological and developmental mechanisms
An understanding of the physiological causes and consequences of the eusocial condition has been somewhat slow; nonetheless, major advancements have been made in learning more about the mechanistic and developmental processes that lead to eusociality.[47]
Involvement of pheromones
Pheromones are thought to play an important role in the physiological mechanisms underlying the development and maintenance of eusociality. The most well-studied queen pheromone system in social insects is that of the honey bee Apis mellifera. Queen mandibular glands were found to produce a mixture of five compounds, three aliphatic and two aromatic, which have been found to control workers.[48] Mandibular gland extracts inhibit workers from constructing queen cells in which new queens are reared which can delay the hormonally based behavioral development of workers and can suppress ovarian development in workers.[47][48] Both behavioral effects mediated by the nervous system often leading to recognition of queens (releaser) and physiological effects on the reproductive and endocrine system (primer) are attributed to the same pheromones. These pheromones volatilize or are deactivated within thirty minutes, allowing workers to respond rapidly to the loss of their queen.[47]
The levels of two of the aliphatic compounds increase rapidly in virgin queens within the first week after eclosion (emergence from the pupal case), which is consistent with their roles as sex attractants during the mating flight.[48] It is only after a queen is mated and begins laying eggs, however, that the full blend of compounds is made.[48] The physiological factors regulating reproductive development and pheromone production are unknown.
In several ant species, reproductive activity has also been associated with pheromone production by queens.[48] In general, mated egg laying queens are attractive to workers whereas young winged virgin queens, which are not yet mated, elicit little or no response. However, very little is known about when pheromone production begins during the initiation of reproductive activity or about the physiological factors regulating either reproductive development or queen pheromone production in ants.[48]
Among ants, the queen pheromone system of the fire ant Solenopsis invicta is particularly well studied. Both releaser and primer pheromones have been demonstrated in this species.[48] A queen recognition (releaser) hormone is stored in the poison sac along with three other compounds. These compounds were reported to elicit a behavioral response from workers.[48] Several primer effects have also been demonstrated. Pheromones initiate reproductive development in new winged females, called female sexuals.[48] These chemicals also inhibit workers from rearing male and female sexuals, suppress egg production in other queens of multiple queen colonies and cause workers to execute excess queens.[47][48] The action of these pheromones together maintains the eusocial phenotype which includes one queen supported by sterile workers and sexually active males (drones). In queenless colonies that lack such pheromones, winged females will quickly shed their wings, develop ovaries and lay eggs. These virgin replacement queens assume the role of the queen and even start to produce queen pheromones.[48] There is also evidence that queen weaver ants Oecophylla longinoda have a variety of exocrine glands that produce pheromones, which prevent workers from laying reproductive eggs.[47]
Similar mechanisms are used for the eusocial wasp species Vespula vulgaris. In order for a Vespula vulgaris queen to dominate all the workers, usually numbering more than 3000 in a colony, she exerts pheromone to signal her dominance. The workers were discovered to regularly lick the queen while feeding her, and the air-borne pheromone from the queen's body alerts those workers of her dominance.[49]
The mode of action of inhibitory pheromones which prevent the development of eggs in workers has been convincingly demonstrated in the bumble bee Bombus terrestris.[47] In this species, pheromones suppress activity of the corpora allata and juvenile hormone (JH) secretion. The corpora allata is an endocrine gland that produces JH, a group of hormones that regulate many aspects of insect physiology.[50] With low JH, eggs do not mature. Similar inhibitory effects of lowering JH were seen in halictine bees and polistine wasps, but not in honey bees.[47]
Other strategies
A variety of strategies in addition to the use of pheromones have evolved that give the queens of different species of social insects a measure of reproductive control over their nest mates.[47] In many Polistes wasp colonies, monogamy is established soon after colony formation by physical dominance interactions among foundresses of the colony including biting, chasing and food soliciting.[47] Such interactions created a dominance hierarchy headed by individuals with the greatest ovarian development.[47] Larger, older individuals often have an advantage during the establishment of dominance hierarchies.[47] The rank of subordinates is positively correlated with the degree of ovarian development and the highest ranking individual usually becomes queen if the established queen disappears.[47] Workers do not oviposit when queens are present because of a variety of reasons: colonies tend to be small enough that queens can effectively dominate workers, queens practice selective oophagy or egg eating, or the flow of nutrients favors queen over workers and queens rapidly lay eggs in new or vacated cells.[47] However, it is also possible that morphological differences favor the worker. In certain species of wasps, such as Apoica flavissima queens are smaller than their worker counterparts. This can lead to interesting worker-queen dynamics, often with the worker policing queen behaviors. Other wasps, like Polistes instabilis have workers with the potential to develop into reproductives, but only in cases where there are no queens to suppress them.
In primitively eusocial bees (where castes are morphologically similar and colonies usually small and short-lived), queens frequently nudge their nest mates and then burrow back down into the nest.[47] This behavior draws workers into the lower part of the nest where they may respond to stimuli for cell construction and maintenance.[47] Being nudged by the queen may play a role in inhibiting ovarian development and this form of queen control is supplemented by oophagy of worker laid eggs.[47] Furthermore, temporally discrete production of workers and gynes (actual or potential queens) can cause size dimorphisms between different castes as size is strongly influenced by the season during which the individual is reared. In many wasp species worker caste determination is characterized by a temporal pattern in which workers precede non-workers of the same generation.[51] In some cases, for example in the bumble bee, queen control weakens late in the season and the ovaries of workers develop to an increasing extent.[47] The queen attempts to maintain her dominance by aggressive behavior and by eating worker laid eggs; her aggression is often directed towards the worker with the greatest ovarian development.[47]
In highly eusocial wasps (where castes are morphologically dissimilar), both the quantity and quality of food seem to be important for caste differentiation.[47] Recent studies in wasps suggest that differential larval nourishment may be the environmental trigger for larval divergence into one of two developmental classes destined to become either a worker or a gyne.[51] All honey bee larvae are initially fed with royal jelly, which is secreted by workers, but normally they are switched over to a diet of pollen and honey as they mature; if their diet is exclusively royal jelly, however, they grow larger than normal and differentiate into queens. This jelly seems to contain a specific protein, designated as royalactin, which increases body size, promotes ovary development and shortens the developmental time period.[52] Furthermore, the differential expression in Polistes of larval genes and proteins (also differentially expressed during queen versus caste development in honey bees) indicate that regulatory mechanisms may occur very early in development.[51]
Definition debates

Subsequent to Wilson's original definition, other authors have sought to narrow or expand the definition of eusociality by focusing on the nature and degree of the division of labor, which was not originally specified. A narrower and more widely accepted definition specifies the requirement for irreversibly distinct behavioral groups or castes (with respect to sterility and/or other features). Such a definition, however, excludes social vertebrates, like the mole rats, and some Hymenoptera species, like the weaver ants.[1] For example, depending on the availability of resources and the condition of the environment, mole rats can change between different types of social behaviors.[33] In 2005, according to Wilson and Hölldobler, these types of animals would be considered primitively eusocial, which is different from eusocial, since the labor division is not permanent.[6] A broader definition, on the other hand, would include mole rats because it allows for any temporary division of labor or non-random distribution of reproductive success to constitute eusociality.
In 2010, Nowak, Tarnita and Wilson challenged the theoretical explanation of the evolution of eusociality. Based on the concept of inclusive fitness, the kin selection theory considers the relatedness of individuals to be one of the most important factors that lead to eusociality. This brought up issues like the irrelevance of haplodiploidy and maternal control. Nowak et al. argued that the kin selection theory is inadequate because it can explain only a subset of eusocial populations due to its assumptions (i.e. "all interactions must be additive and pairwise" which excludes any interaction that involves more than two players). To them, the standard natural selection theory, which is a more general approach than the current explanation of eusociality, is the appropriate theory to use since it explains the same phenomenon and it would work for a larger number of eusocial cases. It also requires simpler mathematical calculations when explaining the evolution of eusociality.[39][53] This paper led to a large influx of publications that refuted Nowak et al.’s ideas and supported the validity and specificity of the kin selection theory. For example, Trivers and Hare studied the haplodiploid Hymenoptera and they found that the workers were able to win the parent-offspring conflict, countering the parents’ best interest and selecting the outcome that has the greater benefit for the workers (i.e. reproductive success with shares higher relatedness to workers than the queen).[54] This refuted Nowak et al.’s defense that future offspring development could be based solely on the fitness of parents.
Another debate focused on whether or not humans may be considered eusocial.[55] In Wilson's book, 2012's The Social Conquest of the Earth, he refers to humans as a species of eusocial ape. He supports his reasoning by stating our eusocial similarities to ants. Humans also fall under Wilson's original criteria of eusociality (division of labor, overlapping generations, and cooperative care of young including ones that are not their own). Through cooperation and teamwork, ants and humans gain a type of "superpower" that is unavailable to other social animals that have failed to make the leap from social to eusocial. Eusociality creates the superorganism.[56] This has caused conflict amongst biologists as not all believe that a term reserved for invertebrates can explain humanity. Others do not believe that humans are eusocial because humans make the decision to be "cooperative" (i.e. babysitting a non-related child) whereas eusociality is a behavioral strategy that is not specifically chosen by an individual.
See also
- Dense heterarchy
- Evolutionarily stable strategy
- Gyne
- Patterns of self-organization in ants
- Presociality
- Reciprocity (social psychology)
- Stigmergy
- Task allocation and partitioning of social insects
- International Union for the Study of Social Insects
References
- 1 2 3 4 5 Crespi, Bernard J.; Douglas Yanega (1995). "The Definition of Eusociality". Behav Ecol 6: 109–115. doi:10.1093/beheco/6.1.109.
- 1 2 Batra, Suzanne W. T. (Jan 1968). "Behavior of Some Social and Solitary Halictine Bees Within Their Nests: A Comparative Study (Hymenoptera: Halictidae)". Journal of the Kansas Entomological Society 41 (1): 120–133.
- 1 2 Michener, Charles D. (1969). "Comparative Social Behavior of Bees". Annu. Rev. Entomol. 14: 299–342. doi:10.1146/annurev.en.14.010169.001503.
- 1 2 Gadagkar, Raghavendra (1993). "And now... eusocial thrips!". Current Science 64 (4): 215–216.
- ↑ Wilson, Edward O. (1971). The Insect Societies. Cambridge. Massachusetts: Belknap Press of Harvard University Press.
- 1 2 3 Wilson, Edward O.; Bert Hölldobler (20 September 2005). "Eusociality: Origin and Consequences". PNAS 102 (38): 13367–13371. doi:10.1073/pnas.0505858102. PMC 1224642. PMID 16157878.
- ↑ Hölldobler, B. (1990). The Ants. Cambridge, MA: Belknap Press.
- ↑ Zara, Fernando; Balestieri, Jose (2000). "Behavioural Catalogue of Polistes versicolor Olivier (Vespidae: Polistinae) Post-emergent Colonies". Naturalia 25: 301–19.
- ↑ Wongvilas, S.; Deowanish, S.; Lim, J.; Xie, V. R. D.; Griffith, O. W.; Oldroyd, B. P. (2010). "Interspecific and conspecific colony mergers in the dwarf honey bees Apis andreniformis and A. florea". Insectes Sociaux 57 (3): 251–255. doi:10.1007/s00040-010-0080-7.
- ↑ Bartareau, T. (1996). "Foraging Behaviour of Trigona Carbonaria (Hymenoptera: Apidae) at Multiple-Choice Feeding Stations". Australian Journal of Zoology 143: 143. doi:10.1071/zo9960143.
- ↑ Conway, John R. "The Biology of Honey Ants." The American Biology Teacher. , Vol. 48, No. 6 (Sep., 1986), pp. 335–343.
- ↑ West-Eberhard, M. J. (1982). "The Nature and Evolution of Swarming In Tropical Social Wasps (Vespidae, Polistinae, Polybini)". Smithsonian Tropical Research Institute.
- ↑ van Veen, J.W.; Sommeijer, M. J.; Meeuwsen F. (November 1997). "Behaviour of drones in Melipona (Apidae, Meliponinae)". Insectes Sociaux 44 (4): 435–447. doi:10.1007/s000400050063.
- ↑ Wcislo, W. T., Wille, A., Orozco, E. (1993). "Nesting biology of tropical solitary and social sweat bees, Lasioglossum (Dialictus) figueresi Wcislo and L. (D.) aeneiventre (Friese) (Hymenoptera: Halictidae)". Insectes Sociaux 40: 21–40. doi:10.1007/BF01338830.
- ↑ Richards, Miriam H. "Evidence for geographic variation in colony social organization in an obligately social sweat bee, Lasioglossum malachurum Kirby (Hymenoptera; Halictidae)". Canadian Journal of Zoology 78 (7): 1259–1266. doi:10.1139/z00-064.
- ↑ Costa-Leonardo AM, Haifig I. (2014). Termite Communication During Different Behavioral Activities. In: Biocommunication of Animals. Dortrecht, Springer, 161-190.
- ↑ Thorne, B. L. (1997). "Evolution of eusociality in termites". Annual Review of Ecology, Evolution and Systematics 28: 27–54. doi:10.1146/annurev.ecolsys.28.1.27.
- ↑ Adams, E.S. (1987). "Territory size and population limits in mangrove termites". Journal of Animal Ecology 56: 1069–1081. doi:10.2307/4967.
- ↑ "Science: The Australian beetle that behaves like a bee". New Scientist. 1992-05-09. Retrieved 2010-10-31.
- 1 2 D. S. Kent & J. A. Simpson (1992). "Eusociality in the beetle Austroplatypus incompertus (Coleoptera: Curculionidae)". Naturwissenschaften 79: 86–87. doi:10.1007/BF01131810.
- ↑ Stern, D.L. (1994). "A phylogenetic analysis of soldier evolution in the aphid family Hormaphididae". Proceedings of the Royal Society 256: 203–209. doi:10.1098/rspb.1994.0071.
- ↑ Aoki, S.; Imai, M. (2005). "Factors affecting the proportion of sterile soldiers in growing aphid colonies". Population Ecology 47: 127–136. doi:10.1007/s10144-005-0218-z.
- ↑ Crespi B. J. (1992). "Eusociality in Australian gall thrips". Nature 359 (6397): 724–726. doi:10.1038/359724a0.
- ↑ Stern, D.; Foster, W. (1996). "The evolution of soldiers in aphids". Biological Reviews 71: 27–79. doi:10.1111/j.1469-185x.1996.tb00741.x.
- ↑ Duffy, J. Emmett; Cheryl L. Morrison; Ruben Rios (2000). "Multiple origins of eusociality among sponge-dwelling shrimps (Synalpheus)". Evolution 54 (2): 503–516. doi:10.1111/j.0014-3820.2000.tb00053.x. PMID 10937227.
- ↑ Duffy, J. E (1998). "On the frequency of eusociality in snapping shrimps (Decapoda: Alpheidae), with description of a second eusocial species". Bulletin of Marine Science 63 (2): 387–400.
- ↑ Duffy, J.E. (2003). "The ecology and evolution of eusociality in sponge-dwelling shrimp". Genes, Behaviors and Evolution of Social Insects: 217–254.
- ↑ Duffy, J.E.; Macdonald, K.S. (2010). "Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis". Proceedings of the Royal Society B-Biological Sciences 277 (1681): 575–584. doi:10.1098/rspb.2009.1483. PMC 2842683. PMID 19889706.
- ↑ Hultgren, K.M.; Duffy, J.E. (2012). "Phylogenetic community ecology and the role of social dominance in sponge-dwelling shrimp". Ecology Letters 15 (7): 704–713. doi:10.1111/j.1461-0248.2012.01788.x.
- ↑ Macdonald, K.S.; Rios, R.; Duffy, J.E. (2006). "Biodiversity, host specificity, and dominance by eusocial species among sponge-dwelling alpheid shrimp on the Belize Barrier Reef". Diversity and Distributions 12 (2): 165–178. doi:10.1111/j.1366-9516.2005.00213.x.
- 1 2 Burda, H. Honeycutt; Begall, S.; Locker-Grutjen, O; Scharff, A. (2000). "Are naked and common mole-rats eusocial and if so, why?". Behavioral Ecology and Sociobiology 47 (5): 293–303. doi:10.1007/s002650050669.
- ↑ Angier, Natalie (April 2012). "Edward O. Wilson’s New Take on Human Nature". Smithsonian Institution. Retrieved 2 October 2015.
As Wilson sees it, human beings are eusocial apes, and in our brand of extreme togetherness we stand apart—from other living monkeys and apes, and from the many hominids that either preceded or coexisted with us and are now extinct
- 1 2 O'Riain, M.J.; Faulkes, C. G. (2008). "African mole rats: eusociality, relatedness and ecological constraints". Ecology of Social Evolution: 207–223. doi:10.1007/978-3-540-75957-7_10.
- ↑ O' Riain, M.; et al. (1996). "A Dispersive Morph in the Naked Mole-Rat". Nature 380 (6575): 619–621. doi:10.1038/380619a0.
- ↑ Williams, S.A. and Shattuck, M.R. (2015). "Ecology, longevity and naked mole-rats: confounding effects of sociality?". Proceedings of the Royal Society of London B: Biological Sciences 282 (1802): 20141664. doi:10.1098/rspb.2014.1664.
- ↑ Thorne, B.L.; Grimaldi, DA & Krishna, K (January 1, 2001) [1st. Pub. 2000]. "Early fossil history of the termites". In Abe, T.; Bignell, D.E & Higashi, M. Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers. pp. 77–93.
- ↑ Darwin, Charles. On the Origin of Species, 1859. Chapter 8
- ↑ Hamilton, W. D. (20 March 1964). "The Genetical Evolution of Social Behaviour II". Journal of Theoretical Biology 7 (1): 17–52. doi:10.1016/0022-5193(64)90039-6. PMID 5875340. Retrieved 13 November 2012.
- 1 2 Nowak, Martin; Corina Tarnita; EO Wilson (26 August 2010). "The evolution of eusociality". Nature 466 (7310): 1057–1062. doi:10.1038/nature09205. PMC 3279739. PMID 20740005. Retrieved 15 Mar 2011.
- ↑ William O. H. Hughes, Benjamin P. Oldroyd, Madeleine Beekman, Francis L. W. Ratnieks (2008-05-30). "Ancestral Monogamy Shows Kin Selection Is Key to the Evolution of Eusociality". Science (American Association for the Advancement of Science) 320 (5880): 1213–1216. doi:10.1126/science.1156108. PMID 18511689. Retrieved 2008-08-04.
- ↑ Cahan, SH. & E. Gardner-Morse (2013). "The emergence of reproductive division of labor in forced queen groups of the ant Pogonomyrmex barbatus". Journal of Zoology 291 (1): 12–22. doi:10.1111/jzo.12071.
- ↑ Molteno, A. J., Bennett, N. C. (2002). "Rainfall, dispersal and reproductive inhibition in eusocial Damaraland mole-rats (Cryptomys damarensis)". Journal of Zoology 256: 445–448. doi:10.1017/s0952836902000481.
- ↑ Nowak, M.A.; Tarnita, C.E.; Wilson, E.O. (2010). "The evolution of eusociality". Nature 466 (7310): 1057–1062. doi:10.1038/nature09205. PMC 3279739. PMID 20740005.
- ↑ Abbot, Patrick; et al. (2011). "Inclusive fitness theory and eusociality". Nature (Nature Publishing Group, a division of Macmillan Publishers Limited.) 471 (7339): E1–E4. doi:10.1038/nature09831. PMID 21430721.
- ↑ Zara, Fernando; Balestieri, Jose (2000). "Behavioural Catalogue of Polistes versicolor Olivier (Vespidae: Polistinae) Post-emergent Colonies". Naturalia 25: 301–319.
- ↑ Wongvilas, S.; Deowanish, S; Lim, J.; Xie, V. R. D.; Griffith, O. W.; Oldroyd, B. P. (2010). "Interspecific and conspecific colony mergers in the dwarf honey bees Apis andreniformis and A. florea". Insectes Sociaux 57 (3): 251–255. doi:10.1007/s00040-010-0080-7.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Fletcher, D.; Ross K. (1985). "Regulation of Reproduction in Eusocial Hymenoptera". Annual Review of Entomology 30: 319–343. doi:10.1146/annurev.ento.30.1.319.
- 1 2 3 4 5 6 7 8 9 10 11 Vargo, E. (1999). "Reproductive development and ontogeny or queen pheromone production in the fire ant Solenopsis invicta". Physiological Entomology 24: 370–376. doi:10.1046/j.1365-3032.1999.00153.x.
- ↑ Carpenter, J.M (1987). "Phylogenetic relationships and classification of the Vespinae (Hymenoptera: Vespidae)". Systematic Entomology 12: 413–431. doi:10.1111/j.1365-3113.1987.tb00213.x.
- ↑ Feyereisen, R.; Tobe S. (1981). "A rapid partition assay for routine analysis of juvenile hormone released by insect corpora allata". Analytical Biochemistry 111: 372–375. doi:10.1016/0003-2697(81)90575-3.
- 1 2 3 Hunt, J.; Wolschin F.; Henshaw M.; Newman T.; Toth A.; Amdam G. (17 May 2010). "Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp". PLOS ONE 5: e10674. doi:10.1371/journal.pone.0010674.
- ↑ Kamakura, Masaki (May 2011). "Royalactin induces queen differentiation in honeybees". Nature 473 (7348): 478–483. doi:10.1038/nature10093. PMID 21516106.
- ↑ http://www.wired.com/2010/08/kin-selection-challenged/2/
- ↑ Trivers, RL; Hare H (23 January 1976). "Haploidploidy and the Evolution of the Social Insect". Science 191 (4224): 249–263. doi:10.1126/science.1108197. PMID 1108197.
- ↑ Foster, Kevin R.; Ratnieks, Francis L.W. (2005). "A new eusocial vertebrate?" (PDF). TRENDS in Ecology and Evolution 20 (7): 363–364. doi:10.1016/j.tree.2005.05.005.
- ↑ Wilson, Edward O. (2012). The Social Conquest of Earth. New York: Liveright Pub. Corp.
External links
| ||||||||||||||
| ||||||||||||||

