Skraup reaction
The Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup (1850-1910). In the archetypal Skraup reaction, aniline is heated with sulfuric acid, glycerol, and an oxidizing agent such as nitrobenzene to yield quinoline.[1][2][3][4]
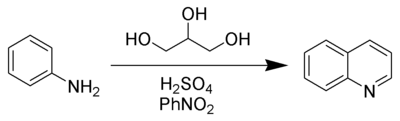
In this example, nitrobenzene serves as both the solvent and the oxidizing agent. The reaction, which otherwise has a reputation for being violent, is typically conducted in the presence of ferrous sulfate.[5] Arsenic acid may be used instead of nitrobenzene and the former is better since the reaction is less violent.[6]
Reaction mechanism
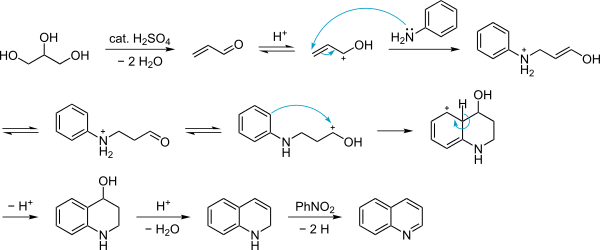
The reaction mechanism is unclear, yet there is good reason to believe that acrolein (obtained by dehydration of glycerol in presence of concentrated sulfuric acid) is an intermediate, which then undergoes 1,4-addition. Acrolein itself is not used since it undergoes polymerisations under the conditions of the experiment.[6]
See also
References
- ↑ Skraup, Z. H. (1880). "Eine Synthese des Chinolins". Berichte 13: 2086.
- ↑ Manske, R. H. F. (1942). "The Chemistry of Quinolines.". Chem. Rev. 30: 113. doi:10.1021/cr60095a006.
- ↑ Manske, R. H. F.; Kulka, M. (1953). Org. React. 7: 80–99. Missing or empty
|title=(help) - ↑ Wahren, M. (1964). "Stabilisotop markierte verbindungen—II , Untersuchung der skraupschen chinolin-synthese mit hilfe von 15N". Tetrahedron 20 (12): 2773. doi:10.1016/S0040-4020(01)98495-9.
- ↑ Clarke, H. T.; Davis, A. W. (1941). "Quinoline". Org. Synth.; Coll. Vol. 1, p. 478
- 1 2 Finar, I. L.; "Organic Chemistry" Volume 1, page 857 (1973)