Serum amyloid P component
| Amyloid P component, serum |
|---|
|

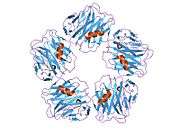
Cartoon model of SAP showing helices in red, sheets in yellow and coils in green. |
| Available structures |
| PDB |
Ortholog search: PDBe, RCSB |
| List of PDB id codes |
|
1GYK, 1LGN, 1SAC, 2A3W, 2A3X, 2A3Y, 2W08, 3D5O, 3KQR, 4AVS, 4AVT, 4AVV, 4AYU
|
|
|
| Identifiers |
|---|
| Symbols |
APCS ; HEL-S-92n; PTX2; SAP |
|---|
| External IDs |
OMIM: 104770 MGI: 98229 HomoloGene: 123932 ChEMBL: 4929 GeneCards: APCS Gene |
|---|
|
|
| RNA expression pattern |
|---|
|
|
|
More reference expression data |
| Orthologs |
|---|
| Species |
Human |
Mouse |
|---|
| Entrez |
325 |
20219 |
|---|
| Ensembl |
ENSG00000132703 |
ENSMUSG00000026542 |
|---|
| UniProt |
P02743 |
P12246 |
|---|
| RefSeq (mRNA) |
NM_001639 |
NM_011318 |
|---|
| RefSeq (protein) |
NP_001630 |
NP_035448 |
|---|
| Location (UCSC) |
Chr 1:
159.59 – 159.59 Mb |
Chr 1:
172.89 – 172.9 Mb |
|---|
| PubMed search |
|
|
|---|
|
The serum amyloid P component (SAP) is the identical serum form of amyloid P component (AP), a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid".[1] APCS is its human gene.[2]
In amyloidosis
AP makes up 14% of the dry mass of amyloid deposits[3] and is thought to be an important contributor to the pathogenesis of a related group of diseases called the Amyloidoses.[4] These conditions are characterised by the ordered aggregation of normal globular proteins and peptides into insoluble fibres which disrupt tissue architecture and are associated with cell death. AP is thought to decorate and stabilise aggregates by preventing proteolytic cleavage and hence inhibiting fibril removal via the normal protein scavenging mechanisms.[5] This association is utilised in the routine clinical diagnostic technique of SAP scintigraphy whereby radio-labelled protein is injected into patients to locate areas of amyloid deposition.[6] The SAP-amyloid association has also been identified as a possible drug target for anti-amyloid therapy, with the recent development and first stage clinical trials of a compound called CPHPC (R-1-[6-[R-2-carboxy-pyrrolidin-1-yl]-6-oxohexanoyl] pyrrolidine-2-carboxylic acid), a small molecule able to strip AP from deposits by reducing levels of circulating SAP.[7]
Structure
SAP is a member of the pentraxins family, characterised by calcium dependent ligand binding and distinctive flattened β-jellyroll structure similar to that of the legume lectins.[8] The name "pentraxin" is derived from the Greek word for five (penta) and berries (ragos) relating to the radial symmetry of five monomers forming a ring approximately 95 Å across and 35 Å deep. Human SAP has 51% sequence homology with C-reactive protein (CRP), a classical acute phase response plasma protein, and is a more distant relative to the "long" pentraxins such as PTX3 (a cytokine modulated molecule) and several neuronal pentraxins. Both SAP and CRP are evolutionary conserved in all vertebrates and also found in distant invertebrates such as the horseshoe crab (Limulus polyphemus).[9]
References
- ↑ Cathcart ES, Shirahama T, Cohen AS (1967). "Isolation and identification of a plasma component of amyloid". Biochim. Biophys. Acta 147: 392–393.
- ↑ "Entrez Gene: APCS amyloid P component, serum".
- ↑ Skinner M, Pepys MB, Cohen AS, Heller LM, Lian JB (1980). Freitas, Antonio Falcão de; Glenner, George G.; Costa, Pedro Pinho e. editors, ed. Amyloid and amyloidosis: proceedings of the Third International Symposium on Amyloidosis, Póvoa de Varzim, Portugal, 23–28 September 1979. Amsterdam: Excerpta Medica. pp. 384–391. ISBN 0-444-90124-8.
- ↑ Botto M, Hawkins PN, Bickerstaff MC, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB (1997). "Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene". Nat. Med. 3 (8): 855–9. doi:10.1038/9544. PMID 9256275.
- ↑ Tennent GA, Lovat LB, Pepys MB (1995). "Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer disease and systemic amyloidosis" (PDF). Proc. Natl. Acad. Sci. U.S.A. 92 (10): 4299–303. doi:10.1073/pnas.92.10.4299. PMC 41931. PMID 7753801.
- ↑ Hawkins PN, Pepys MB (1995). "Imaging amyloidosis with radiolabelled SAP". Eur J Nucl Med 22 (7): 595–9. doi:10.1007/BF01254559. PMID 7498219.
- ↑ Pepys MB, Herbert J, Hutchinson WL, Tennent GA, Lachmann HJ, Gallimore JR, Lovat LB, Bartfai T, Alanine A, Hertel C, Hoffmann T, Jakob-Roetne R, Norcross RD, Kemp JA, Yamamura K, Suzuki M, Taylor GW, Murray S, Thompson D, Purvis A, Kolstoe S, Wood SP, Hawkins PN (2002). "Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis". Nature 417 (6886): 254–9. doi:10.1038/417254a. PMID 12015594.
- ↑ Emsley J, White HE, O'Hara BP, Oliva G, Srinivasan N, Tickle IJ, Blundell TL, Pepys MB, Wood SP (1994). "Structure of pentameric human serum amyloid P component". Nature 367 (6461): 338–45. doi:10.1038/367338a0. PMID 8114934.
- ↑ Pepys MB, Booth DR, Hutchinson WL, Gallimore JR, Collins PM, Hohenester E (1997). "Amyloid P component. A critical review". Amyloid-International Journal of Experimental and Clinical Investigation 4: 274–295.
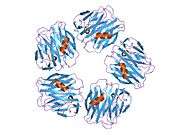
PDB gallery |
|---|
| | 1gyk: SERUM AMYLOID P COMPONENT CO-CRYSTALLISED WITH MOBDG AT NEUTRAL PH |
| 1lgn: DECAMERIC DAMP COMPLEX OF HUMAN SERUM AMYLOID P COMPONENT |
| 1sac: THE STRUCTURE OF PENTAMERIC HUMAN SERUM AMYLOID P COMPONENT |
| 2a3w: Decameric structure of human serum amyloid P-component bound to Bis-1,2-{[(Z)-2-carboxy-2-methyl-1,3-dioxane]-5-yloxycarbamoyl}-ethane |
| 2a3x: Decameric crystal structure of human serum amyloid P-component bound to Bis-1,2-{[(Z)-2carboxy- 2-methyl-1,3-dioxane]- 5-yloxycarbonyl}-piperazine |
| 2a3y: Pentameric crystal structure of human serum amyloid P-component bound to Bis-1,2-{[(Z)-2carboxy-2-methyl-1,3-dioxane]-5-yloxycarbamoyl}-ethane. |
|
|
|