Sertoli cell
| Sertoli cell | |
|---|---|
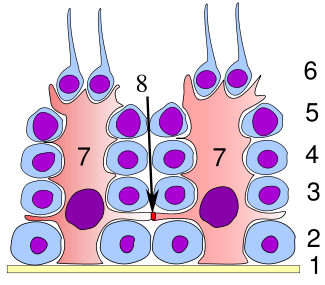 Germinal epithelium of the testicle. 1: basal lamina 2: spermatogonia 3: spermatocyte 1st order 4: spermatocyte 2nd order 5: spermatid 6: mature spermatid 7: Sertoli cell 8: tight junction (blood testis barrier) | |
 Histological section through testicular parenchyma of a boar. 1 Lumen of Tubulus seminiferus contortus 2 spermatids 3 spermatocytes 4 spermatogonia 5 Sertoli cell 6 Myofibroblasts 7 Leydig cells 8 capillaries | |
| Identifiers | |
| MeSH | A05.360.444.849.789 |
A Sertoli cell (a kind of sustentacular cell) is a "nurse" cell of the testicles that is part of a seminiferous tubule and helps in the process of spermatogenesis; that is, the production of sperm.
It is activated by follicle-stimulating hormone (FSH) secreted by the adenohypophysis and has FSH-receptor on its membranes. It is specifically located in the convoluted seminiferous tubules (since this is the only place in the testes where the spermatozoa are produced). Development of Sertoli cells is directed by the testis-determining factor protein.
Functions
Because its main function is to nourish the developing sperm cells through the stages of spermatogenesis, the Sertoli cell has also been called the "mother" or "nurse" cell.[1] Sertoli cells also act as phagocytes, consuming the residual cytoplasm during spermatogenesis. Translocation of germ cells from the base to the lumen of the seminiferous tubules occurs by conformational changes in the lateral margins of the Sertoli cells.
Secretory
Sertoli cells secrete the following substances:
- anti-Müllerian hormone (AMH) — secreted during the early stages of fetal life.
- inhibin and activins — secreted after puberty, and work together to regulate FSH secretion
- androgen binding protein (also called testosterone binding globulin) — increases testosterone concentration in the seminiferous tubules to stimulate spermatogenesis
- estradiol — aromatase from Sertoli cells convert testosterone to 17 beta estradiol to direct spermatogenesis
- glial cell line-derived neurotrophic factor (GDNF) — has been demonstrated to function in promoting undifferentiating spermatogonia, which ensures stem cell self-renewal during the perinatal period.
- ETS Related Molecule or ERM transcription factor ERM transcription factor — needed for maintenance of the spermatogonial stem cell in the adult testis.
- transferrin — a blood plasma protein for iron ion delivery[2]
Structural
The tight junctions of Sertoli cells form the blood-testis barrier, a structure that partitions the interstitial blood compartment of the testis from the adluminal compartment of the seminiferous tubules. Because of the apical progression of the spermatogonia, the tight junctions must be dynamically reformed and broken to allow the immunoidentical spermatogonia to cross through the blood-testis barrier so they can become immunologically unique. Sertoli cells control the entry and exit of nutrients, hormones and other chemicals into the tubules of the testis as well as make the adluminal compartment an immune-privileged site.
The cell is also responsible for establishing and maintaining the spermatogonial stem cell niche, which ensures the renewal of stem cells and the differentiation of spermatogonia into mature germ cells that progress stepwise through the long process of spermatogenesis, ending in the release of spermatozoa. Sertoli cells bind to spermatogonial cells via N-cadherins and galctosyltransferase (via carbohydrate residues).
Other functions
During the maturation phase of spermiogenesis, the Sertoli cells consume the unneeded portions of the spermatozoa.
Production of Sertoli cells
Sertoli cells are required for male sexual development. During male development, the gene SRY activates SOX9, which then activates and forms a feedforward loop with FGF9. Sertoli cell proliferation and differentiation is mainly activated by FGF9.[3] The absence of FGF9 tends to cause a female to develop[4]
Once fully differentiated, the Sertoli cell has been considered to be terminally differentiated, and is unable to proliferate.[5] Therefore, once spermatogenesis has begun, no more Sertoli cells are created.
Recently however, some scientists have found a way to induce Sertoli cells to a juvenile proliferative phenotype outside of the body.[6] This gives rise to the possibility of repairing some defects that cause male infertility.
It has been suggested that they may derive from mesonephros.[7]
Nomenclature
Sertoli cells are called so because of their eponym Enrico Sertoli, an Italian physiologist who discovered them while studying medicine in the University of Pavia, Italy.[8]
He published a description of this cell in 1865. The cell was discovered by Sertoli with a Belthle microscope purchased in 1862, which he used while studying medicine.
In the 1865 publication, his first description used the terms "tree-like cell" or "stringy cell" and most importantly he referred to these "mother cells." It was other scientists who used Enrico's family name, Sertoli, to label these cell in publications, starting in 1888. As of 2006, two textbooks that are devoted specifically to the Sertoli cell have been published.
Histology
On slides, using standard staining, it can be easy to confuse the Sertoli cells with the other cells of the germinal epithelium. The most distinctive feature of the Sertoli cells is the dark nucleolus.[9]
Pathology
Sertoli-Leydig cell tumour are part of the sex cord-stromal tumour group of ovarian neoplasms.
Additional images
See also
References
- ↑ Rato, Luís; Alves, Marco G.; Socorro, Sílvia; Duarte, Ana I.; Cavaco, José E.; Oliveira, Pedro F. (1 May 2012). "Metabolic regulation is important for spermatogenesis". Nature Reviews Urology 9 (6): 330–338. doi:10.1038/nrurol.2012.77. PMID 22549313.
- ↑ Xiong X, Wang A, Liu G, Liu H, Wang C, Xia T, Chen X, Yang K (2006). "Effects of p,p'-dichlorodiphenyldichloroethylene on the expressions of transferrin and androgen-binding protein in rat Sertoli cells". Environ Res 101 (3): 334–9. doi:10.1016/j.envres.2005.11.003. PMID 16380112.
- ↑ Kim, Y.; Kobayashi, A.; Sekido, R.; Dinapoli, L.; Brennan, J.; Chaboissier, M. C.; Poulat, F.; Behringer, R. R.; Lovell-Badge, R.; Capel, B. (2006). "Fgf9 and Wnt4 Act as Antagonistic Signals to Regulate Mammalian Sex Determination". PLoS Biology 4 (6): e187. doi:10.1371/journal.pbio.0040187. PMC 1463023. PMID 16700629.
- ↑ Moniot, Brigitte; Declosmenil, Faustine; Barrionuevo, Francisco; Scherer, Gerd; Aritake, Kosuke; Malki, Safia; Marzi, Laetitia; Cohen-Solal, Ann; Georg, Ina; Klattig, Jürgen; Englert, Christoph; Kim, Yuna; Capel, Blanche; Eguchi, Naomi; Urade, Yoshihiro; Boizet-Bonhoure, Brigitte; Poulat, Francis (2009). "The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation". Development (The Company of Biologists Ltd.) 136 (11): 1813–1821. doi:10.1242/dev.032631. PMID 19429785.
- ↑ Sharpe, R. M.; McKinnell, C; Kivlin, C; Fisher, J. S. (2003). "Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood". Reproduction (Cambridge, England) 125 (6): 769–84. PMID 12773099.
- ↑ Nicholls, P. K.; Stanton, P. G.; Chen, J. L.; Olcorn, J. S.; Haverfield, J. T.; Qian, H; Walton, K. L.; Gregorevic, P; Harrison, C. A. (2012). "Activin signaling regulates Sertoli cell differentiation and function". Endocrinology 153 (12): 6065–77. doi:10.1210/en.2012-1821. PMID 23117933.
- ↑ Peter D. Vize; Adrian S. Woolf; Jonathan Bard (2003). The kidney: from normal development to congenital disease. Academic Press. pp. 82–. ISBN 978-0-12-722441-1. Retrieved 18 November 2010.
- ↑ synd/518 at Who Named It?
- ↑ OSU Center for Veterinary Health Sciences - OSU-CVHS Home
External links
- Histology image: 17805loa – Histology Learning System at Boston University
- Histology image: 17806loa – Histology Learning System at Boston University
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
