Symbiogenesis
Symbiogenesis, or endosymbiotic theory, is an evolutionary theory that explains the origin of eukaryotic cells from prokaryotes. It states that several key organelles of eukaryotes originated as a symbiosis between separate single-celled organisms. According to this theory, mitochondria, plastids (for example chloroplasts), and possibly other organelles representing formerly free-living bacteria (prokaryotes) were taken inside another cell as an endosymbiont around 1.5 billion years ago. Molecular and biochemical evidence suggest that mitochondria developed from proteobacteria (in particular, Rickettsiales, the SAR11 clade,[1][2] or close relatives) and chloroplasts from cyanobacteria (in particular, nitrogen-fixing filamentous cyanobacteria[3][4]).
History
The theory of symbiogenesis (Greek: σύν syn "together", βίωσις biosis "living", and γένεσις genesis "origin or birth") was first articulated by the Russian botanist Konstantin Mereschowsky in 1910,[5] although he described the fundamental elements of the theory in a paper five years earlier.[6][7] Mereschkowski was familiar with work by botanist Andreas Schimper, who had observed in 1883 that the division of chloroplasts in green plants closely resembled that of free-living cyanobacteria, and who had himself tentatively proposed (in a footnote) that green plants had arisen from a symbiotic union of two organisms.[8] In 1918 the French scientist Paul Portier published Les Symbiotes in which he claimed that the mitochondria originated from a symbiosis process.[9]Ivan Wallin extended the idea of an endosymbiotic origin to mitochondria in the 1920s.[10][11] A Russian botanist Boris Kozo-Polyansky was the first to explain the theory in terms of Darwinian evolution.[12] In his 1924 book Symbiogenesis: A New Principle of Evolution he wrote, "The theory of symbiogenesis is a theory of selection relying on the phenomenon of symbiosis."[13] These theories were initially dismissed or ignored. More detailed electron microscopic comparisons between cyanobacteria and chloroplasts (for example studies by Hans Ris published in 1961[14]), combined with the discovery that plastids and mitochondria contain their own DNA[15] (which by that stage was recognized to be the hereditary material of organisms) led to a resurrection of the idea in the 1960s.
The theory was advanced and substantiated with microbiological evidence by Lynn Margulis in a 1967 paper, On the origin of mitosing cells.[16] In her 1981 work Symbiosis in Cell Evolution she argued that eukaryotic cells originated as communities of interacting entities, including endosymbiotic spirochaetes that developed into eukaryotic flagella and cilia. This last idea has not received much acceptance, because flagella lack DNA and do not show ultrastructural similarities to bacteria or archaea (see also: Evolution of flagella and Prokaryotic cytoskeleton). According to Margulis and Dorion Sagan,[17] "Life did not take over the globe by combat, but by networking" (i.e., by cooperation). The possibility that peroxisomes may have an endosymbiotic origin has also been considered, although they lack DNA. Christian de Duve proposed that they may have been the first endosymbionts, allowing cells to withstand growing amounts of free molecular oxygen in the Earth's atmosphere. However, it now appears that they may be formed de novo, contradicting the idea that they have a symbiotic origin.[18]
It is thought that over millennia these endosymbionts transferred some of their own DNA to the host cell's nucleus (called "endosymbiotic gene transfer") during the evolutionary transition from a symbiotic community to an instituted eukaryotic cell. The endosymbiotic theory is considered to be a type of saltational evolution.[19]
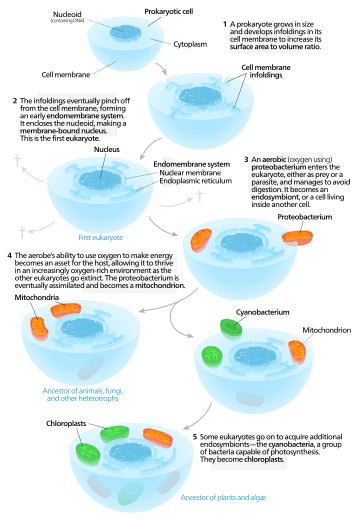
From endosymbionts to organelles
According to Keeling and Archibald,[20] the usual way to distinguish organelles from endosymbionts is by their reduced genome sizes. As an endosymbiont evolves into an organelle, most of their genes are transferred to the host cell genome. The host cell and organelle need to develop a transport mechanism that enables transfer back of the protein products needed by the organelle but now manufactured by the cell. Cyanobacteria and α-proteobacteria are the most closely related free-living organisms to plastids and mitochondria respectively.[21] Both cyanobacteria and α-proteobacteria maintain a large (>6Mb) genome encoding thousands of proteins.[21] Plastids and mitochondria exhibit a dramatic reduction in genome size when compared to their bacterial relatives.[21] Chloroplast genomes in photosynthetic organisms are normally 120-200kb[22] encoding 20-200 proteins[21] and mitochondrial genomes in humans are approximately 16kb and encode 37 genes, 13 of which are proteins.[23] Using the example of the freshwater amoeboid, however, Paulinella chromatophora, which contains chromatophores found to be evolved from cyanobacteria, these authors argue that this is not the only possible criterion; another is that the host cell has assumed control of the regulation of the former endosymbiont's division, thereby synchronizing it with the cell's own division.[20] Nowack and her colleagues[24] performed gene sequencing on the chromatophore (1.02 Mb) and found that only 867 proteins were encoded by these photosynthetic cells. Comparisons with their closest free living cyanobacteria of the genus Synechococcus (having a genome size 3 Mb, with 3300 genes) revealed that chromatophores underwent a drastic genome shrinkage. Chromatophores contained genes that were accountable for photosynthesis but were deficient in genes that could carry out other biosynthetic functions; this observation suggests that these endosymbiotic cells are highly dependent on their hosts for their survival and growth mechanisms. Thus, these chromatophores were found to be non-functional for organelle-specific purposes when compared to mitochondria and plastids. This distinction could have promoted the early evolution of photosynthetic organelles.
The loss of genetic autonomy, that is, the loss of many genes from endosymbionts, occurred very early in evolutionary time.[25] Taking into account the entire original endosymbiont genome, there are three main possible fates for genes over evolutionary time. The first fate involves the loss of functionally redundant genes,[25] in which genes that are already represented in the nucleus are eventually lost.The second fate involves the transfer of genes to the nucleus.[21][25][26][27][28] The loss of autonomy and integration of the endosymbiont with its host can be primarily attributed to nuclear gene transfer.[28] As organelle genomes have been greatly reduced over evolutionary time, nuclear genomes have expanded and become more complex.[21] As a result, many plastid and mitochondrial processes are driven by nuclear encoded gene products.[21] In addition, many nuclear genes originating from endosymbionts have acquired novel functions unrelated to their organelles.[21][28]
The mechanisms of gene transfer are not fully known, however, multiple hypotheses exist to explain this phenomenon. The cDNA hypothesis involves the use of mRNAs to transport genes from organelles to the nucleus where they are converted to cDNA and incorporated into the genome.[21][26] The cDNA hypothesis is based on studies of the genomes of flowering plants.[21] Protein coding RNAs in mitochondria are spliced and edited using organelle-specific splice and editing sites.[21] Nuclear copies of some mitochondrial genes, however, do not contain organelle-specific splice sites, suggesting a processed mRNA intermediate.[21] The cDNA hypothesis has since been revised as edited mitochondrial cDNAs are unlikely to recombine with the nuclear genome and are more likely to recombine with their native mitochondrial genome. If the edited mitochondrial sequence recombines with the mitochondrial genome, mitochondrial splice sites would no longer exist in the mitochondrial genome.[21] Any subsequent nuclear gene transfer would therefore also lack mitochondrial splice sites.[21]
The bulk flow hypothesis is the alternative to the cDNA hypothesis, stating that escaped DNA, rather than mRNA, is the mechanism of gene transfer.[21][26] According to this hypothesis, disturbances to organelles, including autophagy (normal cell destruction), gametogenesis (the formation of gametes), and cell stress, release DNA which is imported into the nucleus and incorporated into the nuclear DNA using non-homologous end joining (repair of double stranded breaks).[26] For example, in the initial stages of endosymbiosis, due to a lack of major gene transfer, the host cell had little to no control over the endosymbiont.[25] The endosymbiont underwent cell division independently of the host cell, resulting in many "copies" of the endosymbiont within the host cell.[25] Some of the endosymbionts lysed (bursted) and high levels of DNA were incorporated into the nucleus.[25] A similar mechanism is thought to occur in tobacco plants, who show a high rate of gene transfer and whose cells contain multiple chloroplasts.[25] In addition, the bulk flow hypothesis is also supported by the presence of non-random clusters of organelle genes, suggesting the simultaneous movement of multiple genes.[26]
Organellar genomes
Plastomes and mitogenomes

The third and final possible fate of endosymbiont genes is that they remain in the organelles. Plastids and mitochondria, although they have lost much of their genomes, retain genes encoding rRNAs, tRNAs, proteins involved in redox reactions and proteins required for transcription, translation and replication.[21][22][25] There are many hypotheses to explain why the organelles retain a small portion of their genome, however no one hypothesis will apply to all organisms[25] and the topic is still quite controversial.[21] The hydrophobicity hypothesis states that highly hydrophobic (water hating) proteins (such as the membrane bound proteins involved in redox reactions) are not easily transported through the cytosol and therefore these proteins must be encoded in their respective organelles.[21][25] The code disparity hypothesis states that the limit on transfer is due to differing genetic codes and RNA editing between the organelle and the nucleus.[25] The redox control hypothesis states that genes encoding redox reaction proteins are retained in order to effectively couple the need for repair and the synthesis of these proteins.[21][22][25] For example, if one of the photosystems is lost from the plastid, the intermediate electron carriers may lose or gain too many electrons, signalling the need for repair of a photosystem.[22] The time delay involved in signalling the nucleus and transporting a cytosolic protein to the organelle results in the production of damaging reactive oxygen species.[21][22][25] The final hypothesis states that the assembly of membrane proteins, particularly those involved in redox reactions, require coordinated synthesis and assembly of subunits, however translation and protein transport coordination is more difficult to control in the cytoplasm.[25]
Non-photosynthetic plastid genomes
The majority of the genes in the mitochondria and plastid are related to the expression (transcription, translation and replication) of genes encoding proteins involved in either photosynthesis (in plastids) or cellular respiration (in mitochondria).[21][22][25] One might predict, that the loss of photosynthesis or cellular respiration would allow for the complete loss of the plastid genome or the mitochondrial genome respectively.[25] While there are numerous examples of mitochondrial descendants (mitosomes and hydrogenosomes) that have lost their entire organellar genome,[29] non-photosynthetic plastids tend to retain a small genome.[25] There are two main hypotheses to explain this occurrence:
The essential tRNA hypothesis: There have been no documented functional plastid to nucleus gene transfers of genes encoding RNA products (tRNAs and rRNAs).[25] As a result, plastids must make their own functional RNAs or import nuclear counterparts.[25] The genes encoding tRNA-Glu and tRNA-fmet, however, appear to be indispensable.[25] The plastid is responsible for haem biosynthesis, which requires plastid encoded tRNA-Glu (from the gene trnE) as a precursor molecule.[25] Like other genes encoding RNAs, trnE cannot be transferred to the nucleus.[25] In addition, it is unlikely trnE could be replaced by a cytosolic tRNA-Glu as trnE is highly conserved; single base changes in trnE have resulted in the loss of haem synthesis.[25] The gene for tRNA-formylmethionine (tRNA-fmet) is also encoded in the plastid genome and is required for translation initiation in both plastids and mitochondria.[25] A plastid is required to continue expressing the gene for tRNA-fmet so long as the mitochondrion is translating proteins.[25]
The limited window hypothesis: This hypothesis offers a more general explanation for the retention of genes in non-photosynthetic plastids.[30] According to the bulk flow hypothesis, genes are transferred to the nucleus following the disturbance of organelles.[26] Disturbance was common in the early stages of endosymbiosis, however, once the host cell gained control of organelle division, eukaryotes could evolve to have only one plastid per cell.[25] Having only one plastid severely limits gene transfer[25] as the lysis of the single plastid would likely result in cell death.[25][30] Consistent with this hypothesis,[30] organisms with multiple plastids show an 80-fold increase in plastid to nucleus gene transfer compared to organisms with single plastids.[30]
Evidence
Evidence that mitochondria and plastids arose from bacteria is as follows:[31][32][33]
- New mitochondria and plastids are formed only through a process similar to binary fission.
- If a cell's mitochondria or chloroplasts are removed, the cell does not have the means to create new ones.[34] For example, in some algae, such as Euglena, the plastids can be destroyed by certain chemicals or prolonged absence of light without otherwise affecting the cell. In such a case, the plastids will not regenerate.
- Transport proteins called porins are found in the outer membranes of mitochondria and chloroplasts, are also found in bacterial cell membrane.[35][36][37]
- A membrane lipid cardiolipin is exclusively found in the inner mitochondrial membrane and bacterial cell membrane.[38]
- Both mitochondria and plastids contain single circular DNA that is different from that of the cell nucleus and that is similar to that of bacteria (both in their size and structure).
- The genomes, including the specific genes, are basically similar between mitochondria and the Rickettsial bacteria.[39]
- Genome comparisons indicate that cyanobacteria contributed to the genetic origin of plastids.[40]
- DNA sequence analysis and phylogenetic estimates suggest that nuclear DNA contains genes that probably came from plastids.
- These organelles' ribosomes are like those found in bacteria (70S).
- Proteins of organelle origin, like those of bacteria, use N-formylmethionine as the initiating amino acid.
- Much of the internal structure and biochemistry of plastids, for instance the presence of thylakoids and particular chlorophylls, is very similar to that of cyanobacteria. Phylogenetic estimates constructed with bacteria, plastids, and eukaryotic genomes also suggest that plastids are most closely related to cyanobacteria.
- Mitochondria have several enzymes and transport systems similar to those of bacteria.
- Some proteins encoded in the nucleus are transported to the organelle, and both mitochondria and plastids have small genomes compared to bacteria. This is consistent with an increased dependence on the eukaryotic host after forming an endosymbiosis. Most genes on the organellar genomes have been lost or moved to the nucleus. Most genes needed for mitochondrial and plastid function are located in the nucleus. Many originate from the bacterial endosymbiont.
- Plastids are present in very different groups of protists, some of which are closely related to forms lacking plastids. This suggests that if chloroplasts originated de novo, they did so multiple times, in which case their close similarity to each other is difficult to explain.
- Many of these protists contain "primary" plastids that have not yet been acquired from other plastid-containing eukaryotes.
- Among eukaryotes that acquired their plastids directly from bacteria (known as Archaeplastida), the glaucophyte algae have chloroplasts that strongly resemble cyanobacteria. In particular, they have a peptidoglycan cell wall between the two membranes.

Secondary endosymbiosis
Primary endosymbiosis involves the engulfment of a bacterium by another free living organism. Secondary endosymbiosis occurs when the product of primary endosymbiosis is itself engulfed and retained by another free living eukaryote. Secondary endosymbiosis has occurred several times and has given rise to extremely diverse groups of algae and other eukaryotes. Some organisms can take opportunistic advantage of a similar process, where they engulf an alga and use the products of its photosynthesis, but once the prey item dies (or is lost) the host returns to a free living state. Obligate secondary endosymbionts become dependent on their organelles and are unable to survive in their absence (for a review see McFadden 2001[41]). RedToL, the Red Algal Tree of Life Initiative funded by the National Science Foundation highlights the role red algae or Rhodophyta played in the evolution of our planet through secondary endosymbiosis.
One possible secondary endosymbiosis in process has been observed by Okamoto & Inouye (2005). The heterotrophic protist Hatena behaves like a predator until it ingests a green alga, which loses its flagella and cytoskeleton, while Hatena, now a host, switches to photosynthetic nutrition, gains the ability to move towards light and loses its feeding apparatus.[42]
The process of secondary endosymbiosis left its evolutionary signature within the unique topography of plastid membranes. Secondary plastids are surrounded by three (in euglenophytes and some dinoflagellates) or four membranes (in haptophytes, heterokonts, cryptophytes, and chlorarachniophytes). The two additional membranes are thought to correspond to the plasma membrane of the engulfed alga and the phagosomal membrane of the host cell. The endosymbiotic acquisition of a eukaryote cell is represented in the cryptophytes; where the remnant nucleus of the red algal symbiont (the nucleomorph) is present between the two inner and two outer plastid membranes.
Despite the diversity of organisms containing plastids, the morphology, biochemistry, genomic organisation, and molecular phylogeny of plastid RNAs and proteins suggest a single origin of all extant plastids – although this theory is still debated.[43][44]
Some species including Pediculus humanus have multiple chromosomes in the mitochondrion. This and the phylogenetics of the genes encoded within the mitochondrion suggest that mitochondria have multiple ancestors, that these were acquired by endosymbiosis on several occasions rather than just once, and that there have been extensive mergers and rearrangements of genes on the several original mitochondrial chromosomes.[45]
See also
- Angomonas deanei, a protozoan that harbours an obligate bacterial symbiont
- Endosymbiont
- Hatena arenicola, a species that appears to be in the process of acquiring an endosymbiont
- Hydrogen hypothesis
- James A. Lake
- Kleptoplasty
- Mixotricha paradoxa, which itself is a symbiont, contains endosymbiotic bacteria
- Numt, abbreviation of "nuclear mitochondrial DNA"
- Parasite Eve, fiction about endosymbiosis
- Midi-chlorian, fictional endosymbionts
- Protocell
- Transfer of mitochondrial and chloroplast DNA to the nucleus
- Viral eukaryogenesis, hypothesis that the cell nucleus originated from endosymbiosis
References
- ↑ "Mitochondria Share an Ancestor With SAR11, a Globally Significant Marine Microbe". ScienceDaily. July 25, 2011. Retrieved 2011-07-26.
- ↑ J. Cameron Thrash; et al. (2011). "Phylogenomic evidence for a common ancestor of mitochondria and the SAR11 clade". Scientific Reports 1: 13. doi:10.1038/srep00013. PMID 22355532.
- ↑ Deusch, O.; et al. (2008). "Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor". Mol. Biol. Evol 25: 748–761. doi:10.1093/molbev/msn022.
- ↑ Ochoa de Alda, JAG; Esteban, R; Diago, ML; Houmard, J. "The plastid ancestor originated among one of the major cyanobacterial lineages". Nature Communications 5: 4937. doi:10.1038/ncomms5937.
- ↑ Mereschkowsky, Konstantin (1910). "Theorie der zwei Plasmaarten als Grundlage der Symbiogenesis, einer neuen Lehre von der Ent‐stehung der Organismen.". Biol Centralbl. 30: 353‐367.
- ↑ Mereschkowski C (1905). "Über Natur und Ursprung der Chromatophoren im Pflanzenreiche". Biol Centralbl 25: 593–604.
- ↑ Martin, William; Mayo Roettger; Thorsten Kloesges; Thorsten Thiergart; Christian Woehle; Sven Gould; Tal Dagan. "Modern endosymbiotic theory: Getting lateral gene transfer in-to the equation" (PDF). Journal of Endocytobiosis and Cell Research 23: 1–5.(journal URL: )
- ↑ Schimper AFW (1883). "Über die Entwicklung der Chlorophyllkörner und Farbkörper". Bot. Zeitung 41: 105–14, 121–31, 137–46, 153–62.
- ↑ Lane, Nick (2005). Power, Sex, Suicide. Mitochondria and the Meaning of Life. New York: Oxford University Press. p. 14. ISBN 9780199205646.
- ↑ Wallin IE (1923). "The Mitochondria Problem". The American Naturalist 57 (650): 255–61. doi:10.1086/279919.
- ↑ Wallin, I.E. (1927). Symbionticism and the origin of species. Baltimore: Williams & Wilkins Company. p. 171.
- ↑ Margulis, Lynn (2011). "Symbiogenesis. A new principle of evolution rediscovery of Boris Mikhaylovich Kozo-Polyansky (1890–1957)". Paleontological Journal 44 (12): 1525–1539. doi:10.1134/S0031030110120087.
- ↑ Corning, Peter A. (2010). Holistic Darwinism: Synergy, Cybernetics, and the Bioeconomics of Evolution. Chicago: University of Chicago Press. p. 81. ISBN 978-0-22611-633-4.
- ↑ Ris H, Singh RN (January 1961). "Electron microscope studies on blue-green algae". J Biophys Biochem Cytol 9 (1): 63–80. doi:10.1083/jcb.9.1.63. PMC 2224983. PMID 13741827.
- ↑ Stocking C and Gifford E (1959). "Incorporation of thymidine into chloroplasts of Spirogyra". Biochem. Biophys. Res. Comm. 1 (3): 159–64. doi:10.1016/0006-291X(59)90010-5.
- ↑ Lynn Sagan (1967). "On the origin of mitosing cells". J Theor Biol 14 (3): 255–274. doi:10.1016/0022-5193(67)90079-3. PMID 11541392.
- ↑ Margulis, Lynn; Sagan, Dorion (2001). "Marvellous microbes". Resurgence 206: 10–12.
- ↑ Gabaldón T, Snel B, van Zimmeren F, Hemrika W, Tabak H, Huynen MA (2006). "Origin and evolution of the peroxisomal proteome". Biol. Direct 1 (1): 8. doi:10.1186/1745-6150-1-8. PMC 1472686. PMID 16556314. (Provides evidence that contradicts an endosymbiotic origin of peroxisomes. Instead it is suggested that they evolutionarily originate from the Endoplasmic Reticulum)
- ↑ Michael Syvanen, Clarence I. Kado Horizontal Gene Transfer Academic Press, p. 405 ISBN 978-0126801262
- 1 2 Keeling, P. J.; Archibald, J.M. (2008). "Organelle evolution: what’s in a name?". Current Biology 18: 345–347. doi:10.1016/j.cub.2008.02.065. PMID 18430636.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Timmis, Jeremy N.; Ayliffe, Michael A.; Huang, Chun Y.; Martin, William. "Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes". Nature Reviews Genetics 5 (2): 123–135. doi:10.1038/nrg1271.
- 1 2 3 4 5 6 Lila Koumandou, V.; Nisbet, R. Ellen R.; Barbrook, Adrian C.; Howe, Christopher J. (2004-01-05). "Dinoflagellate chloroplasts – where have all the genes gone?". Trends in Genetics 20 (5): 261–267. doi:10.1016/j.tig.2004.03.008. ISSN 0168-9525. PMID 15109781.
- ↑ Taanman, Jan-Willem (1999-02-09). "The mitochondrial genome: structure, transcription, translation and replication". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1410 (2): 103–123. doi:10.1016/S0005-2728(98)00161-3.
- ↑ Nowack, E.C.; Melkonian, M.; Glockner, G. (2008). "Chromatophore genome sequence of Paulinella sheds light on acquisition of photosynthesis by eukaryotes.". Current Biology 18: 410–418. doi:10.1016/j.cub.2008.02.051. PMID 18356055.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 Barbrook, Adrian C.; Howe, Christopher J.; Purton, Saul (2006-01-02). "Why are plastid genomes retained in non-photosynthetic organisms?". Trends in Plant Science 11 (2): 101–108. doi:10.1016/j.tplants.2005.12.004. ISSN 1360-1385. PMID 16406301.
- 1 2 3 4 5 6 Leister, Dario (2005-01-12). "Origin, evolution and genetic effects of nuclear insertions of organelle DNA". Trends in Genetics 21 (12): 655–663. doi:10.1016/j.tig.2005.09.004. ISSN 0168-9525. PMID 16216380.
- ↑ Keeling, Patrick J. (2004-10-01). "Diversity and evolutionary history of plastids and their hosts". American Journal of Botany 91 (10): 1481–1493. doi:10.3732/ajb.91.10.1481. ISSN 0002-9122. PMID 21652304.
- 1 2 3 Archibald, John M. "The Puzzle of Plastid Evolution". Current Biology 19 (2): R81–R88. doi:10.1016/j.cub.2008.11.067. ISSN 0960-9822. PMID 19174147.
- ↑ Howe, Christopher J. "Cellular Evolution: What's in a Mitochondrion?". Current Biology 18 (10): R429–R431. doi:10.1016/j.cub.2008.04.007. ISSN 0960-9822. PMID 18492476.
- 1 2 3 4 Lane, Nick (2011-01-01). "Plastids, Genomes, and the Probability of Gene Transfer". Genome Biology and Evolution 3: 372–374. doi:10.1093/gbe/evr003. ISSN 1759-6653. PMC 3101016. PMID 21292628.
- ↑ Kimball, J. 2010. Kimball's Biology Pages. Accessed October 13, 2010. An online open source biology text by Harvard professor, and author of a general biology text, John W. Kimball.
- ↑ Reece, J., Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson, 2010. Campbell Biology. 9th Edition Benjamin Cummings; 9th Ed. (October 7, 2010)
- ↑ Raven, P., George Johnson, Kenneth Mason, Jonathan Losos, Susan Singer, 2010. Biology. McGraw-Hill 9th Ed. (January 14, 2010)
- ↑ Wise, Robert R; Hoober,, J. Kenneth (2007). Structure and function of plastids. Berlin: Springer. p. 104. ISBN 9781402065705.
- ↑ Fischer K, Weber A, Brink S, Arbinger B, Schünemann D, Borchert S, Heldt HW, Popp B, Benz R, Link TA (1994). "Porins from plants. Molecular cloning and functional characterization of two new members of the porin family". J Biol Chem 269 (41): 25754–25760. PMID 7523392.
- ↑ Zeth K, Thein M (2010). "Porins in prokaryotes and eukaryotes: common themes and variations". Biochem J 431 (1): 13–22. doi:10.1042/BJ20100371. PMID 20836765.
- ↑ Fairman JW, Noinaj N, Buchanan SK (2011). "The structural biology of β-barrel membrane proteins: a summary of recent reports". Current Opinion in Structural Biology 21 (4): 523–531. doi:10.1016/j.sbi.2011.05.005. PMC 3164749. PMID 21719274.
- ↑ Mileykovskaya E, Dowhan W (2009). "Cardiolipin membrane domains in prokaryotes and eukaryotes". Biochim Biophys Acta 1788 (10): 2084–2091. doi:10.1016/j.bbamem.2009.04.003. PMC 2757463. PMID 19371718.
- ↑ Andersson SG, Zomorodipour A, Andersson JO, Sicheritz-Pontén T, Alsmark UC, Podowski RM, Näslund AK, Eriksson AS, Winkler HH, Kurland CG (1998). "The genome sequence of Rickettsia prowazekii and the origin of mitochondria". Nature 396 (6707): 133–140. doi:10.1038/24094. PMID 9823893.
- ↑ Dagan T, Roettger M, Stucken K, Landan G, Koch R, Major P, Gould SB, Goremykin VV, Rippka R, Tandeau de Marsac N, Gugger M, Lockhart PJ, Allen JF, Brune I, Maus I, Pühler A, Martin WF (2013). "Genomes of Stigonematalean cyanobacteria (subsection V) and the evolution of oxygenic photosynthesis from prokaryotes to plastids". Genome Biol Evol 5 (1): 31–44. doi:10.1093/gbe/evs117. PMC 3595030. PMID 23221676.
- ↑ McFadden GI (2001). "Primary and secondary endosymbiosis and the origin of plastids". J Phycology 37 (6): 951–9. doi:10.1046/j.1529-8817.2001.01126.x.
- ↑ Okamoto, Noriko; Inouye, Isao (2005). "A Secondary Symbiosis in Progress?" (PDF). Science 310 (5746): 287. doi:10.1126/science.1116125. PMID 16224014. Retrieved 15 February 2015.
- ↑ McFadden GI, van Dooren GG (July 2004). "Evolution: red algal genome affirms a common origin of all plastids". Curr. Biol. 14 (13): R514–6. doi:10.1016/j.cub.2004.06.041. PMID 15242632.
- ↑ Gould SB, Waller RF, McFadden GI (2008). "Plastid evolution". Annu Rev Plant Biol 59 (1): 491–517. doi:10.1146/annurev.arplant.59.032607.092915. PMID 18315522.
- ↑ Georgiades, K. and Raoult, D. (2011). "The rhizome of Reclinomonas americana, Homo sapiens, Pediculus humanus and Saccharomyces cerevisiae mitochondria". Biology Direct 6: 55. doi:10.1186/1745-6150-6-55.
Further reading
- Alberts, Bruce (2002). Molecular biology of the cell. New York: Garland Science. ISBN 0-8153-3218-1. (General textbook)
- Blanchard JL, Lynch M (July 2000). "Organellar genes: why do they end up in the nucleus?". Trends Genet. 16 (7): 315–20. doi:10.1016/S0168-9525(00)02053-9. PMID 10858662. (Discusses theories on how mitochondria and chloroplast genes are transferred into the nucleus, and also what steps a gene needs to go through in order to complete this process.)
- Jarvis P (April 2001). "Intracellular signalling: the chloroplast talks!". Curr. Biol. 11 (8): R307–10. doi:10.1016/S0960-9822(01)00171-3. PMID 11369220. (Recounts evidence that chloroplast-encoded proteins affect transcription of nuclear genes, as opposed to the more well-documented cases of nuclear-encoded proteins that affect mitochondria or chloroplasts.)
- Brinkman FS, Blanchard JL, Cherkasov A, et al. (August 2002). "Evidence that plant-like genes in Chlamydia species reflect an ancestral relationship between Chlamydiaceae, cyanobacteria, and the chloroplast". Genome Res. 12 (8): 1159–67. doi:10.1101/gr.341802. PMC 186644. PMID 12176923.
- Okamoto N, Inouye I (October 2005). "A secondary symbiosis in progress?". Science 310 (5746): 287. doi:10.1126/science.1116125. PMID 16224014.
- Cohen WD, Gardner RS (1959). "Viral Theory and Endosymbiosis" (PDF). (Discusses theory of origin of eukaryotic cells by incorporating mitochondria and chloroplasts into anaerobic cells with emphasis on 'phage bacterial and putative viral mitochondrial/chloroplast interactions.)
- Understanding Science Team. "Cells within cells: An extraordinary claim with extraordinary evidence" (PDF). Understanding Science. University of California, Berkeley. Retrieved 16 February 2014.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
