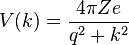Screening effect
In solids, especially in metals and semiconductors, the electrostatic screening or screening effect reduces the electrostatic field and Coulomb potential of an ion inside the solid. Like the electric field of the nucleus is reduced inside an atom or ion due to the shielding effect, the electric fields of ions in conducting solids are further reduced by the cloud of conduction electrons. The screened Coulomb potential is expressed as[1]
where Z is the atomic number, e is the elementary unit charge, r is the distance to the nucleus of the embedded ion, and q is the screening parameter that determines the range of the potential. The screening parameter q plays an important role in theoretical models in solid-state physics.[2] The screened electrostatic potential, like the Yukawa potential, has a simple Fourier transform, expressed as
The screened potential determines the inter atomic force and the phonon dispersion relation in metals. The screened potential is used to calculate the electronic band structure of a large variety of materials, often in combination with pseudopotential models.
See also
References
- ↑ C. Kittel (1953–1976). Introduction to Solid State Physics. Wiley & Sons. ISBN 0-471-49024-5.
- ↑ W. Jones, N. H. March (1973). Theoretical Solid State Physics. Wiley and Sons - Dover Publications. ISBN 0-486-65015-4.
![V(r) = \frac{Z e}{r} \exp[{-q r}]](../I/m/657831de929405abeed45465c899dfa8.png)