Ketene

A ketene is an organic compound of the form R'R''C=C=O. The term is also used specifically to mean ethenone, the simplest ketene, where R' and R'' are hydrogen atoms.
Ketenes were first studied as a class by Hermann Staudinger.[1]
Formation
Ethenone, the simplest ketene, can be formed by pyrolysis (thermal cracking) of acetone:
- CH3−CO−CH3 + ΔT → CH2=C=O + CH4
This reaction is called the Schmidlin ketene synthesis.[2][3]
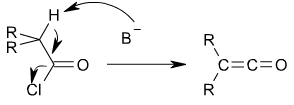
Ketenes can be prepared from acyl chlorides by an elimination reaction in which HCl is lost:
In this reaction, a base, usually triethylamine, removes the acidic proton alpha to the carbonyl group, inducing the formation of the carbon-carbon double bond and the loss of a chloride ion.
Ketenes can also be formed from α-diazoketones by Wolff rearrangement.
Reactions
Ketenes are generally very reactive, and participate in various cycloadditions. They will also undergo [2+2] cycloaddition reactions with electron-rich alkynes to form cyclobutenones, or carbonyl groups to form beta-lactones. With imines beta-lactams are formed. This is the Staudinger synthesis, a facile route to this important class of compounds.
Reactions between diols (HO–R–OH) and bis-ketenes (O=C=CH–R'–CH=C=O) yield polyesters with a repeat unit of (–O–R–O–CO–R'–CO–).
Ethyl acetoacetate, a very important starting material in organic synthesis, can be prepared using a diketene in reaction with ethanol. They directly form ethyl acetoacetate, and the yield is high when carried out under controlled circumstances; this method is therefore used industrially.
See also
References
- ↑ Hermann Staudinger (1905). "Ketene, eine neue Körperklasse" [Ketenes, a new class of substances]. Berichte der deutschen chemischen Gesellschaft 38 (2): 1735–1739. doi:10.1002/cber.19050380283.
- ↑ Ketene in Organic Syntheses Organic Syntheses, Submitted by C. D. Hurd Checked by Oliver Kamm Coll. Vol. 1, p.330 (1941); Vol. 4, p.39 (1925). Link
- ↑ Julius Schmidlin and Maximilian Bergman (1910) "Darstellung des Ketens aus Aceton" (Preparation of ketene from acetone), Berichte der deutschen chemischen Gesellschaft, 43 (3) : 2821-2823.
|