SMK box riboswitch
| SMK box translational riboswitch | |||||
|---|---|---|---|---|---|
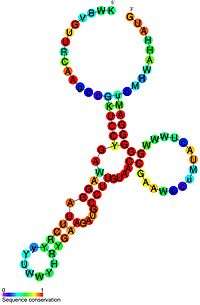 | |||||
| A representation of the SMK box translational riboswitch secondary structure including a colour scheme that indicates the degree of sequence conservation. | |||||
| Identifiers | |||||
| Symbol | SMK_box_riboswitch | ||||
| Alt. Symbols | SAM-III, SMK | ||||
| Rfam | RF01767 | ||||
| Other data | |||||
| RNA type | Riboswitch | ||||
| Domain(s) | Firmicutes | ||||
| |||||
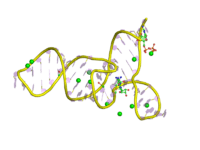
The SMKbox riboswitch (also known as SAM-III) is a RNA element that regulates gene expression in bacteria.[2][3] The SMK box riboswitch is found in the 5' UTR of the MetK gene in lactic acid bacteria. The structure of this element changes upon binding to S-adenosyl methionine (SAM) to a conformation that blocks the shine-dalgarno sequence and blocks translation of the gene.
There are other known SAM-binding riboswitches such as SAM-I and SAM-II, but these appear to share no similarity in sequence or structure to SAM-III.
Structure
The crystal structure of the riboswitch from E. faecalis was solved by X-ray crystallography. The structure showed that the most conserved nucleotides involved in SAM binding were organised around a junction between three helices.[1] In some species there are large insertions of up to 210 nucleotides within this structure.
See also
- SAH riboswitch
- SAM-I riboswitch
- SAM-II riboswitch
- SAM-IV riboswitch
- SAM-V riboswitch
- SAM-Chlorobi RNA motif
- SAM-SAH riboswitch
References
- 1 2 Lu C, Smith AM, Fuchs RT, Ding F, Rajashankar K, Henkin TM, Ke A (2008). "Crystal structures of the SAM-III/S(MK) riboswitch reveal the SAM-dependent translation inhibition mechanism.". Nat Struct Mol Biol 15 (10): 1076–83. doi:10.1038/nsmb.1494. PMC 3467307. PMID 18806797.
- ↑ Fuchs RT, Grundy FJ, Henkin TM (2006). "The S(MK) box is a new SAM-binding RNA for translational regulation of SAM synthetase". Nat. Struct. Mol. Biol. 13 (3): 226–33. doi:10.1038/nsmb1059. PMID 16491091.
- ↑ Fuchs RT, Grundy FJ, Henkin TM (2007). "S-adenosylmethionine directly inhibits binding of 30S ribosomal subunits to the SMK box translational riboswitch RNA". Proc. Natl. Acad. Sci. U.S.A. 104 (12): 4876–80. doi:10.1073/pnas.0609956104. PMC 1829232. PMID 17360376.