SB-612,111
 | |
| Systematic (IUPAC) name | |
|---|---|
|
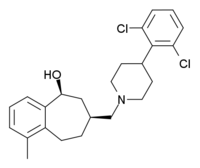
(5S,7S)-7-{[4-(2,6-dichlorophenyl)piperidin-1-yl]methyl}-1-methyl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-5-ol | |
| Identifiers | |
| CAS Number |
371980-98-2 |
| PubChem | CID 10047612 |
| IUPHAR/BPS | 1693 |
| ChemSpider |
8223175 |
| ChEMBL |
CHEMBL559569 |
| Synonyms | SB-612,111 |
| Chemical data | |
| Formula | C24H29Cl2NO |
| Molar mass | 418.398 g/mol |
| |
| |
| | |
SB-612,111 is an opioid receptor ligand which is a potent and selective antagonist for the nociceptin receptor (ORL-1), several times more potent than the older drug J-113,397.[1] It does not have analgesic effects in its own right, but prevents the development of hyperalgesia,[2] and also shows antidepressant effects in animal studies.[3]
See also
References
- ↑ Spagnolo, B; Carrà, G; Fantin, M; Fischetti, C; Hebbes, C; McDonald, J; Barnes, TA; Rizzi, A; et al. (2007). "Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 (−)-cis-1-methyl-7-4-(2,6-dichlorophenyl)piperidin-1-ylmethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol: in vitro studies". The Journal of Pharmacology and Experimental Therapeutics 321 (3): 961–7. doi:10.1124/jpet.106.116764. PMID 17329552.
- ↑ Zaratin, PF; Petrone, G; Sbacchi, M; Garnier, M; Fossati, C; Petrillo, P; Ronzoni, S; Giardina, GA; Scheideler, MA (2004). "Modification of nociception and morphine tolerance by the selective opiate receptor-like orphan receptor antagonist (−)-cis-1-methyl-7-4-(2,6-dichlorophenyl)piperidin-1-ylmethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol (SB-612111)". The Journal of Pharmacology and Experimental Therapeutics 308 (2): 454–61. doi:10.1124/jpet.103.055848. PMID 14593080.
- ↑ Rizzi, A; Gavioli, EC; Marzola, G; Spagnolo, B; Zucchini, S; Ciccocioppo, R; Trapella, C; Regoli, D; Calò, G (2007). "Pharmacological characterization of the nociceptin/orphanin FQ receptor antagonist SB-612111 (−)-cis-1-methyl-7-4-(2,6-dichlorophenyl)piperidin-1-ylmethyl-6,7,8,9-tetrahydro-5H-benzocyclohepten-5-ol: in vivo studies". The Journal of Pharmacology and Experimental Therapeutics 321 (3): 968–74. doi:10.1124/jpet.106.116780. PMID 17329551.
This article is issued from Wikipedia - version of the Wednesday, January 27, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.