S100A10
S100 calcium-binding protein A10 (S100A10), also known as p11, is a protein[1] that is encoded by the S100A10 gene in humans and the S100a10 gene in other species.[2][3] S100A10 is a member of the S100 family of proteins containing two EF-hand calcium-binding motifs. S100 proteins are localized in the cytoplasm and/or nucleus of a wide range of cells. They regulate a number of cellular processes such as cell cycle progression and differentiation. The S100 protein is implicated in exocytosis and endocytosis by reorganization of F-actin.[3]
The p11 protein is linked with the transport of neurotransmitters. Found in the brain of humans and other mammals, it has been implicated in the regulation of mood. In addition, due to its interaction with serotonin-signaling proteins and its correlation with symptoms of mood disorders, p11 is a new potential target for drug therapy.[4]
Gene
The S100 gene family includes at least 13 members that are located as a cluster on chromosome 1q21.[5] In humans, 19 family members are currently known, with most S100 genes (S100A1 to S100A16).
Structure
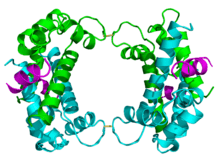
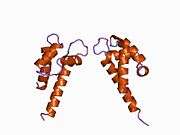
The p11 protein can be found as a free monomer, a homodimer, or a heterotetramer composed of a p11 dimer complex with two molecules of annexin II. The homodimer or heterotetramer can, in turn, dimerize through formation of two disulfide bonds (see figure to the left). The p11 monomer is an asymmetric protein composed of four alpha helices. The dimerized form of the protein is created by packing between the H1 and H4 helices in an antiparallel arrangement with the hydrophobic regions residing in the core.
The structure of p11 is classified by a pair of the helix-loop-helix motif, also known as the EF-hand-type that recognizes and binds calcium ions. This is common to all known S-100 proteins. The EF-hand types, united by an anti-parallel beta-strand between loops L1 and L3, are located on the same side of the molecule, opposite the N-and C-termini.[6] As a member of the S-100 family, its structure resembles that of the S-100A1 and S-100B proteins. This class of proteins has been implicated in the regulation of cytoskeleton assembly, cytosolic enzymes, and membrane dynamics.
P11's involvement with the cytoskeleton may aid the transport of other proteins throughout the cell and to the cell membrane. Unlike other S-100 proteins, the second EF-hand of protein p11 is incapable of binding calcium due to a series of mutations caused by deletions and substitutions. Annexin II, which is attracted to negatively charged phospholipids, binds to p11 at the Ca2+ binding site. In addition, Annexin II has been implicated in membrane-cytoskeleton interactions and in regulations of ion currents and substances across the membrane.[6] P11 and annexin II form a heterotetrameric protein complex that imitates the structure and function of S-100 proteins activated by the binding of calcium. This tetrameric complex is more stable than the p11 dimer, therefore the overexpression of the annexin II gene results in higher levels of p11 protein.[6][7]
Function
P11 is an integral part of cellular structural scaffolding that interacts with plasma membrane proteins through its association with annexin II. Recently, it was discovered to form a complex with annexin I though the mechanism remains unknown. It works together with cytosolic and peripheral membrane-associated proteins such as AHNAK in the development of the intracellular membrane. P11 has been implicated in the transportation of proteins involved in mood regulation, nociception, and cell polarization. It is found in cell types throughout the body though it is located predominantly in the lungs and kidneys. It is involved in the trafficking of proteins to the plasma membrane and can be expressed on the cell surface as a receptor. Many of the transported proteins are cell surface receptors in signal transduction pathways and ion channels. P11 facilitates nociception, Ca2+ uptake, and cell polaraization. Complexed with the annexin II, p11 binds receptor and channel proteins and guides them to the cell surface, resulting in increased membrane localization and consequent magnified functional expression of these proteins.[8]
Ion channels are among the several proteins that are transported through the interaction with p11. Some of these proteins include Nav1.8, TRPV5, TRPV6, TASK-1, and ASIC1a. Nav1.8 is a tetrodotoxin-resistant sodium channel that replaces lost sodium after cell damage. Increased expression of these channels alters the magnitude of the sodium current across the membrane. TRPV5 and TRPV6 are transient receptor potential channels selective for Ca+ and Mg2+ ions. TASK-1 is a two-pore domain K+ channel TWIK-related acid-sensitive K (TASK). P11 can also function as a retention factor, preventing TASK-1 from leaving the endoplasmic reticulum. ASIC1a is an acid-sensing ion channel involved in the pain sensory pathway, which is regulated by p11.[8]
Although the exact mechanism is unclear, p11 protein has shown to be essential in the regulation of serotonin signaling in the brain. Serotonin (5-hydroxytryptamine or 5-HT), is a neurotransmitter found in the central and peripheral nervous systems. It is involved in mechanisms responsible for memory formation and learning, but is most known for its role in the regulation muscle contraction, appetite, sleep, and mood. Varying levels of serotonin found in the brain are associated with the development of mood disorders, such as clinical depression. P11 interacts with the serotonin receptor proteins, 5-HT receptors such as 5-HT1B, modulating the receptor signal transduction pathways activated by the binding of serotonin. P11 also recruits the cell surface expression of the 5-HT4 receptor, increasing its concentration at the synapse. This results in more rapid serotonin-dependent activities. 5-HT4 is involved in the regulation of kinase activity in the central nervous system, phosphorylating target proteins, and facilitating endosomal activities. P11 is coexpressed with 5-HT4 mRNA and its protein in parts of the brain associated with depression, suggesting that their functions are linked and influence mood.[9]
Protein p11 can also be presented on the cell surface as a receptor for tissue-type plasminogen activator (tPA) and plasminogen.[10] Plasmin production by many cells is dependent on p11.
Interactions
S100A10 has been shown to interact with TRPV5,[11] TRPV6, TASK-1, ASIC1a, CTSB,[12] BAD,[13] KCNK3,[14] UBC[15] and ANXA2.[6][15]
There is a specificity in the interaction between p11 and 5-HT1B. In a two-hybrid screen using twenty six out of 29 double-positive prey clones containing the gene encoding p11. This study showed that p11 interacted with 5-HT1B receptors but not with 5-HT1A, 5-HT2A, 5-HT5A, 5-HT6, dopamine D1 or D2 receptors, two irrelevant baits (C{Delta}115 and pRP21), or the empty plasmid.[16] The specific interaction has been verified in three other ways: In HeLa cells and brain tissue p11 was found to coimmunoprecipitate with 5-HT1B receptors; Immunofluorescence studies show colocalization between p11 and 5-HT1B receptors at the cell surface; and distribution of p11 mRNA in the brain resembles that of 5-HT1B receptor mRNA. The table below shows the proteins that interact with p11 and the functional role of p11 in these interactions[17]
Table 1
| Interactor | Biological function of P11 | Reference |
|---|---|---|
| Annexin 2 | Regulation of endosomal functions | [10] |
| 5-HT1B receptor | Localization of 5-HT1B receptors at the cell surface | [16] |
| NaV1.8 sodium channel | Increase of NaV1.8 channels at the plasma membrane | [18] |
| TASK-1 potassium channel | Regulation of TASK-1 channels at the plasma membrane | [14] |
| ASIC-1 channels | Increase of ASIC channels at the plasma membrane | [19] |
| TRPV5/TRPV6 channels | Increase of TRPV5/TRPV6 channels at the plasma membrane | [11] |
| NS3 | Mediation of virus release | [20] |
| Cytosolic phospholipase A2 | Reduced arachidonic acid release | [21] |
| BAD | Inhibition of pro-apoptotic effect | [13] |
| HPV16 L2 | Facilitates binding and entry of human papillomavirus type 16 | [22] |
Regulation
Regulation of Protein Activity
The p11 and annexin II complex is regulated by the phosphorylation of SerII on the annexin II molecule by protein kinase C (PKC). This phosphorylation hinders the complex's ability to bind to certain target molecules. Protein Kinase A (PKA) reverses the effects of PKC by activating a phosphatase, which reactivates the complex through dephosphorylation.[8]
Regulation of Transcription
Current experiments on animals have shown that various factors and physiological stimuli have been successful in regulating the levels of p11 protein transcription. Some of these factors are shown in the table below.[17]
Table 2
| Factor | Biological system | Reference |
|---|---|---|
| Dexamethasone | BEAS and HeLa cells | [23] |
| Transforming growth factor-α | RGM-1 cells | [24] |
| Epidermal growth factor | depolarization BEAS and HeLa cells | [25] |
| Nitric Oxide donors | BEAS and HeLa cells | [26] |
| Interferon-gamma | BEAS cells | [27] |
| Vitamin D | mouse kidney | [11] |
| Retinoic acid | BEAS cells | [18] |
| nerve growth factor | PC12 cells, rat dorsal root ganglion | [28] |
| imipramine | mouse frontal cortex | [16] |
| tranylcypromine | mouse frontal cortex | [16] |
| Electroconvulsive treatment | rat frontal cortex | [16] |
| Sciatic nerve lesion | rat | [29] |
| Experimental autoimmune encephalomyelitis | rat cerebellum | [30] |
Clinical Significance
Depression
Depression is a widespread, debilitating disease affecting persons of all ages and backgrounds. Depression is characterized by a plethora of emotional and physiological symptoms including feelings of sadness, hopelessness, pessimism, guilt, a general loss of interest in life, and a sense of reduced emotional well-being or low energy. Very little is known about the underlying pathophysiology of clinical depression and other related mood-disorders including anxiety, bipolar disorder, ADD, ADHD, and Schizophrenia.
The p11 protein has been intimately linked to mood disorders, to be specific, depression, due to its role in serotonin systems via its interactions with serotonin 5-HT receptors. Serotonin affects diverse systems including the cardiovascular, renal, immune, and gastrointestinal systems. Current research focuses on the neurotransmitter's relationship with mood-regulation.[9]
Under experimentation, mice deficient in the p11 protein display depression-like behaviors. Knockout experiments in which the gene coding for protein p11 was deleted from the mouse genome caused them to show signs of depression. This is also observed in humans. On the other hand, those with sufficient amount of p11 protein behave normally. When mice that showed depressive symptoms were administered anti-depressant drugs, their levels of p11 were found to increase at the same rate, as antidepressants affected their behavioral changes. In addition, post-mortem comparisons of brain tissues showed much lower levels of p11 in depressed compared to control subjects. Levels of p11 have been found to be substantially lower in depressed humans and helpless mice, which suggests that altered p11 levels may be involved in the development of depression-like symptoms.
Treatment
Most of the current drugs and treatments for depression and anxiety increase levels of serotonin transmission among neurons. Selective Serotonin Reuptake Inhibitors (SSRIs), a very successful class of drugs, are known to increase the amount of serotonin available to brain cells quite rapidly. Despite this, their therapeutic effects take a period of several weeks to months. Recent studies show that protein p11 increases the concentration of the serotonin 5-HT receptors at neuronal synapses, thereby rendering serotonin signaling much more efficient. The interaction with the serotonin 1b receptor (5-HT1B) and p11 can be summarized as follows: When p11 levels increases, the number of 5-HT1B receptors on the cell surface increase proportionately.[4] An increase in the number of 5-HT1B receptors on the surface of the neuron increase the effectiveness of serotonin communication across the synapse. On the other hand, when p11 levels decrease, fewer 5-HT1B receptors migrate from inside the neuron to the cell membrane at the synaptic cleft, thus lowering the efficiency with which serotonin signaling can occur across the synapse. These findings suggest that, although the serotonin levels are immediately introduced via medication, the period of time within which the medicine alleviates the patient’s depression most likely relies on other regulatory proteins. Thus, given protein p11’s interaction with serotonin 5-HT receptors and the increasing evidence of the protein’s correlation to mood disorders, this protein has been identified as a target for research in the development of future antidepressants.[31]
Treatment with antidepressants (a tricyclic and monoamine oxidase inhibitor) and electroconvulsive therapy (ECT) caused an increase in the amount of p11 in the brain of these mice - the same biochemical change.[4] The levels of the p11 protein in humans and mice with symptoms of depression were substantially lower in comparison to the levels of p11 in non-depressed animals. Leading researcher Paul Greengard and his colleagues hypothesized that increasing p11 levels would result in the mice exhibiting antidepressant-like behaviors, and the opposite if p11 protein levels were reduced. They used a test that is used to measure antidepressant-like activity to affirm this hypothesis. In their findings, over-expressed p11 genes, compared to the control mice, had increased mobility and more 5-HT1B receptors at the cell surface, which made possible more serotonin transmission. When researchers "knocked out" the p11 gene in mice, they found that the knockout mice had fewer receptors at the cell surface, reduced serotonin signaling, reduced responsiveness to sweet reward, and decreased mobility, behaviors all characteristic of depression-like behaviors. Also, the 5-HT1B receptors of p11 knockout mice were less responsive to serotonin and antidepressant drugs compared to those of control mice, which further implicates p11 in the main action of antidepressant medications.[16] Antidepressant manipulations increase the p11 levels, whereas depressant manipulations reduce it. Therefore, in order to achieve an anti-depression effect, antidepressant medications should focus on the main action of the p11 proteins and increase levels of the protein.[16]
Future Clinical trials
At the current time, a study by the National Institutes of Health Clinical Center (CC) is recruiting participants for a study that will compare levels of p11 protein in people with and without Major Depressive Disorder (MDD) and determine whether p11 levels in patients are affected by treatment with citalopram (Celexa), a serotonin reuptake inhibitor. If successful, a more personalized treatment of MDD will be available in the future.[32]
References
- ↑ Gerke V, Weber K (Nov 1985). "The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells". The EMBO Journal 4 (11): 2917–20. PMC 554598. PMID 2998764.
- ↑ Harder T, Kube E, Gerke V (Apr 1992). "Cloning and characterization of the human gene encoding p11: structural similarity to other members of the S-100 gene family". Gene 113 (2): 269–74. doi:10.1016/0378-1119(92)90406-F. PMID 1533380.
- 1 2 "Entrez Gene: S100A10 S100 calcium binding protein A10".
- 1 2 3 Rosack J (2006). "Protein Discovery May Lead To New Psychiatric Drugs". Psychiatr News.
- ↑ Volz A, Korge BP, Compton JG, Ziegler A, Steinert PM, Mischke D (Oct 1993). "Physical mapping of a functional cluster of epidermal differentiation genes on chromosome 1q21". Genomics 18 (1): 92–9. doi:10.1006/geno.1993.1430. PMID 8276421.
- 1 2 3 4 5 Réty S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, Russo-Marie F, Lewit-Bentley A (Jan 1999). "The crystal structure of a complex of p11 with the annexin II N-terminal peptide". Nature Structural Biology 6 (1): 89–95. doi:10.1038/4965. PMID 9886297.
- ↑ Puisieux A, Ji J, Ozturk M (Jan 1996). "Annexin II up-regulates cellular levels of p11 protein by a post-translational mechanisms". The Biochemical Journal. 313 313 (1): 51–5. doi:10.1042/bj3130051. PMC 1216908. PMID 8546709.
- 1 2 3 Rescher U, Gerke V (Jan 2008). "S100A10/p11: family, friends and functions" (PDF). Pflügers Archiv 455 (4): 575–82. doi:10.1007/s00424-007-0313-4. PMID 17638009.
- 1 2 Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P (Feb 2009). "Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation". The Journal of Neuroscience 29 (6): 1937–46. doi:10.1523/JNEUROSCI.5343-08.2009. PMID 19211900.
- 1 2 Kwon M, MacLeod TJ, Zhang Y, Waisman DM (Jan 2005). "S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors". Frontiers in Bioscience 10 (1-3): 300–25. doi:10.2741/1529. PMID 15574370.
- 1 2 3 van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, Gerke V, Staub O, Nilius B, Bindels RJ (Apr 2003). "Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex". The EMBO Journal 22 (7): 1478–87. doi:10.1093/emboj/cdg162. PMC 152906. PMID 12660155.
- ↑ Mai J, Finley RL, Waisman DM, Sloane BF (Apr 2000). "Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells". The Journal of Biological Chemistry 275 (17): 12806–12. doi:10.1074/jbc.275.17.12806. PMID 10777578.
- 1 2 Hsu SY, Kaipia A, Zhu L, Hsueh AJ (Nov 1997). "Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11". Molecular Endocrinology 11 (12): 1858–67. doi:10.1210/me.11.12.1858. PMID 9369453.
- 1 2 Girard C, Tinel N, Terrenoire C, Romey G, Lazdunski M, Borsotto M (Sep 2002). "p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1". The EMBO Journal 21 (17): 4439–48. doi:10.1093/emboj/cdf469. PMC 125412. PMID 12198146.
- 1 2 He KL, Deora AB, Xiong H, Ling Q, Weksler BB, Niesvizky R, Hajjar KA (Jul 2008). "Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11". The Journal of Biological Chemistry 283 (28): 19192–200. doi:10.1074/jbc.M800100200. PMC 2443646. PMID 18434302.
- 1 2 3 4 5 6 7 Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, Vaugeois JM, Nomikos GG, Greengard P (Jan 2006). "Alterations in 5-HT1B receptor function by p11 in depression-like states". Science 311 (5757): 77–80. doi:10.1126/science.1117571. PMID 16400147.
- 1 2 Falk W, Leonard EJ (May 1982). "Chemotaxis of purified human monocytes in vitro: lack of accessory cell requirement". Infection and Immunity 36 (2): 591–7. doi:10.1016/j.coph.2006.10.001. PMC 351269. PMID 7085073.
- 1 2 Gladwin MT, Yao XL, Cowan M, Huang XL, Schneider R, Grant LR, Logun C, Shelhamer JH (Dec 2000). "Retinoic acid reduces p11 protein levels in bronchial epithelial cells by a posttranslational mechanism". American Journal of Physiology. Lung Cellular and Molecular Physiology 279 (6): L1103–9. PMID 11076800.
- ↑ Donier E, Rugiero F, Okuse K, Wood JN (Nov 2005). "Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a". The Journal of Biological Chemistry 280 (46): 38666–72. doi:10.1074/jbc.M505981200. PMID 16169854.
- ↑ Beaton AR, Rodriguez J, Reddy YK, Roy P (Oct 2002). "The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release". Proceedings of the National Academy of Sciences of the United States of America 99 (20): 13154–9. doi:10.1073/pnas.192432299. PMC 130602. PMID 12235365.
- ↑ Wu T, Angus CW, Yao XL, Logun C, Shelhamer JH (Jul 1997). "P11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85-kDa cytosolic phospholipase A2". The Journal of Biological Chemistry 272 (27): 17145–53. doi:10.1074/jbc.272.27.17145. PMID 9202034.
- ↑ Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, Isas JM, Langen R, Kast WM (2012-01-01). "The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection". PLOS ONE 7 (8): e43519. doi:10.1371/journal.pone.0043519. PMC 3425544. PMID 22927980.
- ↑ Yao XL, Cowan MJ, Gladwin MT, Lawrence MM, Angus CW, Shelhamer JH (Jun 1999). "Dexamethasone alters arachidonate release from human epithelial cells by induction of p11 protein synthesis and inhibition of phospholipase A2 activity". The Journal of Biological Chemistry 274 (24): 17202–8. doi:10.1074/jbc.274.24.17202. PMID 10358078.
- ↑ Akiba S, Hatazawa R, Ono K, Hayama M, Matsui H, Sato T (Nov 2000). "Transforming growth factor-alpha stimulates prostaglandin generation through cytosolic phospholipase A(2) under the control of p11 in rat gastric epithelial cells". British Journal of Pharmacology 131 (5): 1004–10. doi:10.1038/sj.bjp.0703637. PMC 1572404. PMID 11053223.
- ↑ Huang XL, Pawliczak R, Cowan MJ, Gladwin MT, Madara P, Logun C, Shelhamer JH (Oct 2002). "Epidermal growth factor induces p11 gene and protein expression and down-regulates calcium ionophore-induced arachidonic acid release in human epithelial cells". The Journal of Biological Chemistry 277 (41): 38431–40. doi:10.1074/jbc.M207406200. PMID 12163506.
- ↑ Pawliczak R, Cowan MJ, Huang X, Nanavaty UB, Alsaaty S, Logun C, Shelhamer JH (Nov 2001). "p11 expression in human bronchial epithelial cells is increased by nitric oxide in a cGMP-dependent pathway involving protein kinase G activation". The Journal of Biological Chemistry 276 (48): 44613–21. doi:10.1074/jbc.M104993200. PMID 11571284.
- ↑ Huang XL, Pawliczak R, Yao XL, Cowan MJ, Gladwin MT, Walter MJ, Holtzman MJ, Madara P, Logun C, Shelhamer JH (Mar 2003). "Interferon-gamma induces p11 gene and protein expression in human epithelial cells through interferon-gamma-activated sequences in the p11 promoter". The Journal of Biological Chemistry 278 (11): 9298–308. doi:10.1074/jbc.M212704200. PMID 12645529.
- ↑ Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN (Jun 2002). "Annexin II light chain regulates sensory neuron-specific sodium channel expression". Nature 417 (6889): 653–6. doi:10.1038/nature00781. PMID 12050667.
- ↑ De León M, Van Eldik LJ, Shooter EM (Jun 1991). "Differential regulation of S100 beta and mRNAs coding for S100-like proteins (42A and 42C) during development and after lesion of rat sciatic nerve". Journal of Neuroscience Research 29 (2): 155–62. doi:10.1002/jnr.490290204. PMID 1890696.
- ↑ Craner MJ, Lo AC, Black JA, Baker D, Newcombe J, Cuzner ML, Waxman SG (Mar 2003). "Annexin II/p11 is up-regulated in Purkinje cells in EAE and MS". NeuroReport 14 (4): 555–8. doi:10.1097/01.wnr.0000061018.47393.38. PMID 12657884.
- ↑ Hamilton J (2006). "Study Sheds Light on How Depression Drugs Work". National Public Radio.
- ↑ "p11 Protein Levels in Patients With Major Depressive Disorder Treated With Citalopram". ClinicalTrials.gov.
Further reading
- Akiba S, Hatazawa R, Ono K, Hayama M, Matsui H, Sato T (Nov 2000). "Transforming growth factor-alpha stimulates prostaglandin generation through cytosolic phospholipase A(2) under the control of p11 in rat gastric epithelial cells". British Journal of Pharmacology 131 (5): 1004–10. doi:10.1038/sj.bjp.0703637. PMC 1572404. PMID 11053223.
- Gladwin MT, Yao XL, Cowan M, Huang XL, Schneider R, Grant LR, Logun C, Shelhamer JH (Dec 2000). "Retinoic acid reduces p11 protein levels in bronchial epithelial cells by a posttranslational mechanism". American Journal of Physiology. Lung Cellular and Molecular Physiology 279 (6): L1103–9. PMID 11076800.
- Huang XL, Pawliczak R, Cowan MJ, Gladwin MT, Madara P, Logun C, Shelhamer JH (Oct 2002). "Epidermal growth factor induces p11 gene and protein expression and down-regulates calcium ionophore-induced arachidonic acid release in human epithelial cells". The Journal of Biological Chemistry 277 (41): 38431–40. doi:10.1074/jbc.M207406200. PMID 12163506.
- Huang XL, Pawliczak R, Yao XL, Cowan MJ, Gladwin MT, Walter MJ, Holtzman MJ, Madara P, Logun C, Shelhamer JH (Mar 2003). "Interferon-gamma induces p11 gene and protein expression in human epithelial cells through interferon-gamma-activated sequences in the p11 promoter". The Journal of Biological Chemistry 278 (11): 9298–308. doi:10.1074/jbc.M212704200. PMID 12645529.
- Masiakowski P, Shooter EM (Feb 1988). "Nerve growth factor induces the genes for two proteins related to a family of calcium-binding proteins in PC12 cells". Proceedings of the National Academy of Sciences of the United States of America 85 (4): 1277–81. doi:10.1073/pnas.85.4.1277. PMC 279750. PMID 3422491.
- Yao XL, Cowan MJ, Gladwin MT, Lawrence MM, Angus CW, Shelhamer JH (Jun 1999). "Dexamethasone alters arachidonate release from human epithelial cells by induction of p11 protein synthesis and inhibition of phospholipase A2 activity". The Journal of Biological Chemistry 274 (24): 17202–8. doi:10.1074/jbc.274.24.17202. PMID 10358078.
- Schäfer BW, Heizmann CW (Apr 1996). "The S100 family of EF-hand calcium-binding proteins: functions and pathology". Trends in Biochemical Sciences 21 (4): 134–40. doi:10.1016/S0968-0004(96)80167-8. PMID 8701470.
- Dooley TP, Weiland KL, Simon M (Jul 1992). "cDNA sequence of human p11 calpactin I light chain". Genomics 13 (3): 866–8. doi:10.1016/0888-7543(92)90171-N. PMID 1386341.
- Creutz CE, Moss S, Edwardson JM, Hide I, Gomperts B (Apr 1992). "Differential recognition of secretory vesicles by annexins. European Molecular Biology Organization Course "Advanced Techniques for Studying Secretion"". Biochemical and Biophysical Research Communications 184 (1): 347–52. doi:10.1016/0006-291X(92)91199-Z. PMID 1533123.
- Kube E, Weber K, Gerke V (Jun 1991). "Primary structure of human, chicken, and Xenopus laevis p11, a cellular ligand of the Src-kinase substrate, annexin II". Gene 102 (2): 255–9. doi:10.1016/0378-1119(91)90086-Q. PMID 1831433.
- Becker T, Weber K, Johnsson N (Dec 1990). "Protein-protein recognition via short amphiphilic helices; a mutational analysis of the binding site of annexin II for p11". The EMBO Journal 9 (13): 4207–13. PMC 552202. PMID 2148288.
- Schäfer BW, Wicki R, Engelkamp D, Mattei MG, Heizmann CW (Feb 1995). "Isolation of a YAC clone covering a cluster of nine S100 genes on human chromosome 1q21: rationale for a new nomenclature of the S100 calcium-binding protein family". Genomics 25 (3): 638–43. doi:10.1016/0888-7543(95)80005-7. PMID 7759097.
- Kato S, Sekine S, Oh SW, Kim NS, Umezawa Y, Abe N, Yokoyama-Kobayashi M, Aoki T (Dec 1994). "Construction of a human full-length cDNA bank". Gene 150 (2): 243–50. doi:10.1016/0378-1119(94)90433-2. PMID 7821789.
- Engelkamp D, Schäfer BW, Mattei MG, Erne P, Heizmann CW (Jul 1993). "Six S100 genes are clustered on human chromosome 1q21: identification of two genes coding for the two previously unreported calcium-binding proteins S100D and S100E". Proceedings of the National Academy of Sciences of the United States of America 90 (14): 6547–51. doi:10.1073/pnas.90.14.6547. PMC 46969. PMID 8341667.
- Jost M, Gerke V (Oct 1996). "Mapping of a regulatory important site for protein kinase C phosphorylation in the N-terminal domain of annexin II". Biochimica Et Biophysica Acta 1313 (3): 283–9. doi:10.1016/0167-4889(96)00101-2. PMID 8898866.
- Munz B, Gerke V, Gillitzer R, Werner S (Mar 1997). "Differential expression of the calpactin I subunits annexin II and p11 in cultured keratinocytes and during wound repair". The Journal of Investigative Dermatology 108 (3): 307–12. doi:10.1111/1523-1747.ep12286470. PMID 9036930.
- Kang HM, Kassam G, Jarvis SE, Fitzpatrick SL, Waisman DM (Feb 1997). "Characterization of human recombinant annexin II tetramer purified from bacteria: role of N-terminal acetylation". Biochemistry 36 (8): 2041–50. doi:10.1021/bi962569b. PMID 9047302.
- Wu T, Angus CW, Yao XL, Logun C, Shelhamer JH (Jul 1997). "P11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85-kDa cytosolic phospholipase A2". The Journal of Biological Chemistry 272 (27): 17145–53. doi:10.1074/jbc.272.27.17145. PMID 9202034.
- Hsu SY, Kaipia A, Zhu L, Hsueh AJ (Nov 1997). "Interference of BAD (Bcl-xL/Bcl-2-associated death promoter)-induced apoptosis in mammalian cells by 14-3-3 isoforms and P11". Molecular Endocrinology 11 (12): 1858–67. doi:10.1210/me.11.12.1858. PMID 9369453.
- Ramalingam R, Rafii S, Worgall S, Hackett NR, Crystal RG (Dec 1999). "Induction of endogenous genes following infection of human endothelial cells with an E1(-) E4(+) adenovirus gene transfer vector". Journal of Virology 73 (12): 10183–90. PMC 113071. PMID 10559334.
- Mai J, Finley RL, Waisman DM, Sloane BF (Apr 2000). "Human procathepsin B interacts with the annexin II tetramer on the surface of tumor cells". The Journal of Biological Chemistry 275 (17): 12806–12. doi:10.1074/jbc.275.17.12806. PMID 10777578.
External links
| |||||||||||