Retinol
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
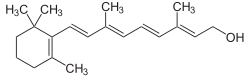
(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraen-1-ol | |
| Identifiers | |
| 68-26-8 | |
| ChEBI | CHEBI:17336 |
| ChEMBL | ChEMBL986 |
| ChemSpider | 393012 |
| 4053 | |
| Jmol interactive 3D | Image |
| PubChem | 1071 |
| UNII | 81G40H8B0T |
| |
| |
| Properties | |
| C20H30O | |
| Molar mass | 286.46 g·mol−1 |
| Melting point | 62–64 °C |
| Boiling point | 137–138 °C (1x10-6 mm Hg) |
| Pharmacology | |
| ATC code | A11 D10AD02, R01AX02, S01XA02 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Retinol is one of the animal forms of vitamin A. It is a diterpenoid and an alcohol. It is convertible to other forms of vitamin A, and the retinyl ester derivative of the alcohol serves as the storage form of the vitamin in animals.
When converted to the retinal (retinaldehyde) form, vitamin A is essential for vision, and when converted to retinoic acid is essential for skin health, teeth remineralization and bone growth. These chemical compounds are collectively known as retinoids, and possess the structural motif of all-trans retinol as a common feature in their structure. Structurally, all retinoids also possess a β-ionone ring and a polyunsaturated side chain, with either an alcohol, aldehyde, a carboxylic acid group or an ester group. The side chain is composed of four isoprenoid units, with a series of conjugated double bonds which may exist in trans- or cis-configuration.[1]
Retinol is produced in the body from the hydrolysis of retinyl esters, and from the reduction of retinal. Retinol in turn is ingested in a precursor form; animal sources (liver and eggs) contain retinyl esters, whereas plants (carrots, spinach) contain provitamin A carotenoids (these may also be considered simply vitamin A). Hydrolysis of retinyl esters results in retinol, while provitamin A carotenoids can be cleaved to produce retinal by carotene dioxygenase in the intestinal mucosa. Retinal, also known as retinaldehyde, can be reversibly reduced to produce retinol or it can be irreversibly oxidized to produce retinoic acid, which then cannot function as the vitamin in the eye.
Commercial production of retinol typically requires retinal synthesis through reduction of a pentadiene derivative and subsequent acidification/hydrolysis of the resulting isomer to produce retinol. Pure retinol is extremely sensitive to oxidization and is prepared and transported at low temperatures and oxygen free atmospheres. When prepared as a dietary supplement, retinol is stabilized as the ester derivatives retinyl acetate or retinyl palmitate.
Discovery
In 1913, Elmer McCollum, a biochemist at the University of Wisconsin–Madison, and colleague Marguerite Davis identified a fat-soluble nutrient in butterfat and cod liver oil. Their work confirmed that of Thomas Osborne and Lafayette Mendel, at Yale, which suggested a fat-soluble nutrient in butterfat, also in 1913.[2] Vitamin A was first synthesized in 1947 by two Dutch chemists, David Adriaan van Dorp and Jozef Ferdinand Arens.
Although the vitamin A was not identified until the 20th century, written observations of conditions created by deficiency of this nutrient appeared much earlier in history. Sommer (2008) classified historical accounts related to vitamin A and/or manifestations of deficiency as follows: "Ancient" accounts; 18th- to 19th-century clinical descriptions (and their purported etiologic associations); early 20th-century laboratory animal experiments, and clinical and epidiomologic observations that identified the existence of this unique nutrient and manifestations of its deficiency.[3]
Chemical structure and function
Many different geometric isomers of retinol, retinal and retinoic acid are possible as a result of either a trans or cis configuration of four of the five double bonds found in the polyene chain. The cis isomers are less stable and can readily convert to the all-trans configuration (as seen in the structure of all-trans-retinol shown here). Nevertheless, some cis isomers are found naturally and carry out essential functions. For example, the 11-cis-retinal isomer is the chromophore of rhodopsin, the vertebrate photoreceptor molecule. Rhodopsin is composed of the 11-cis-retinal covalently linked via a Schiff base to the opsin protein (either rod opsin or blue, red or green cone opsins). The process of vision relies on the light-induced isomerisation of the chromophore from 11-cis to all-trans resulting in a change of the conformation and activation of the photoreceptor molecule. One of the earliest signs of vitamin A deficiency is night-blindness followed by decreased visual acuity.
George Wald won the 1967 Nobel Prize in Physiology or Medicine for his work with retina pigments (also called visual pigments), which led to the understanding of the role of vitamin A in vision.
Many of the non-visual functions of vitamin A are mediated by retinoic acid, which regulates gene expression by activating nuclear retinoic acid receptors.[4] The non-visual functions of vitamin A are essential in the immunological function, reproduction and embryonic development of vertebrates as evidenced by the impaired growth, susceptibility to infection and birth defects observed in populations receiving suboptimal vitamin A in their diet.
Biosynthesis of retinol
Retinol is synthesized from the breakdown of β-carotene. First the β-carotene 15-15’-monooxygenase cleaves β-carotene at the central double bond, creating an epoxide. This epoxide is then attacked by water creating two hydroxyl groups in the center of the structure. The cleavage occurs when these alcohols are reduced to the aldehydes using NADH. This compound is called retinal. Retinal is then reduced to retinol by the enzyme retinol dehydrogenase. Retinol dehydrogenase is an enzyme that is dependent on NADH.[5]

Biological role
Role in embryology
Retinoic acid via the retinoic acid receptor influences the process of cell differentiation, hence, the growth and development of embryos. During development there is a concentration gradient of retinoic acid along the anterior-posterior (head-tail) axis. Cells in the embryo respond to retinoic acid differently depending on the amount present. For example, in vertebrates the hindbrain transiently forms eight rhombomers and each rhombomere has a specific pattern of genes being expressed. If retinoic acid is not present the last four rhombomeres do not develop. Instead rhombomeres 1–4 grow to cover the same amount of space as all eight would normally occupy. Retinoic acid has its effects by turning on a differential pattern of Hox genes which encode different homeodomain transcription factors which in turn can turn on cell type specific genes. Deletion of the Hox-1 gene from rhombomere 4 makes the neurons growing in that region behave like neurons from rhombomere 2. Retinoic acid is not required for patterning of the retina as originally proposed, but retinoic acid synthesized in the retina is secreted into surrounding mesenchyme where it is required to prevent overgrowth of perioptic mesenchyme which can cause microphthalmia, defects in the cornea and eyelid, and rotation of the optic cup.[4]
Stem cell biology
Retinoic acid is an influential factor used in differentiation of stem cells to more committed fates, echoing retinoic acid's importance in natural embryonic developmental pathways. It is thought to initiate differentiation into a number of different cell lineages by unsequestering certain sequences in the genome.
It has numerous applications in the experimental induction of stem cell differentiation; amongst these are the differentiation of human embryonic stem cells to posterior foregut lineages and also to functional motor neurons.
Vision
Vitamin A is converted by the protein RPE65 within the retinal pigment epithelium into 11-cis-retinal. This molecule is then transported into the photoreceptor cells of the retina, where it acts as a light-activated molecular switch within opsin proteins that activates a complex cascade called the visual cycle. This cycle begins with 11-cis retinal absorbing light and isomerizing into all-trans retinal. The change in shape of the molecule after absorbing light in turn changes the configuration of the complex protein rhodopsin, the visual pigment used in low light levels. This represents the first step of the visual cycle. This is why eating foods rich in vitamin A is often said to allow an individual to see in the dark, although the effect they have on one's vision is negligible. In fact, excess vitamin A intake can cause toxicity to the optic nerve and permanent vision loss.
Epithelial cells
Vitamin A is essential for the correct functioning of epithelial cells. In vitamin A deficiency, mucus-secreting cells are replaced by keratin producing cells, leading to xerosis.
Glycoprotein synthesis
Glycoprotein synthesis requires adequate vitamin A status. In severe vitamin A deficiency, lack of glycoproteins may lead to corneal ulcers or liquefaction.
Immune system
Vitamin A is essential to maintain intact epithelial tissues as a physical barrier to infection; it is also involved in maintaining a number of immune cell types from both the innate and acquired immune systems. These include the lymphocytes (B-cells, T-cells, and natural killer cells), as well as many myelocytes (neutrophils, macrophages, and myeloid dendritic cells).
Formation of red blood cells (haematopoiesis)
Vitamin A may be needed for normal haematopoiesis;[6] deficiency causes abnormalities in iron metabolism.[7]
Growth
Vitamin A affects the production of human growth hormone.[8]
Clinical use
All retinoid forms of vitamin A are used in cosmetic (especially in anti-aging and stretch mark creams) and medical applications applied to the skin. Retinoic acid, termed Tretinoin in clinical usage, is used in the treatment of acne and keratosis pilaris in a topical cream. An isomer of tretinoin, isotretinoin is also used orally (under the trade names Accutane and Roaccutane), generally for severe or recalcitrant acne.
Tretinoin, under the alternative name of all-trans retinoic acid (ATRA), is used as chemotherapy for acute promyelocytic leukemia, a subtype of acute myelogenous leukemia. This is because cells of this subtype of leukemia are sensitive to agonists of the retinoic acid receptors (RARs).
Units of measurement
When referring to dietary allowances or nutritional science, retinol is usually measured in international units (IU). IU refers to biological activity and therefore is unique to each individual compound, however 1 IU of retinol is equivalent to approximately 0.3 micrograms (300 nanograms).
Nutrition
| Vitamin properties | |
|---|---|
| Solubility | Fat |
| RDA (adult male) | 900 µg/day |
| RDA (adult female) | 700 µg/day |
| RDA upper limit (adult male) | 3,000 µg/day |
| RDA upper limit (adult female) | 3,000 µg/day |
| Deficiency symptoms | |
| |
| Excess symptoms | |
| |
| Common sources | |
| |
This vitamin plays an essential role in vision, particularly night vision, normal bone and tooth development, reproduction, and the health of skin and mucous membranes (the mucus-secreting layer that lines body regions such as the respiratory tract). Vitamin A also acts in the body as an antioxidant, a protective chemical that may reduce the risk of certain cancers.
There are two sources of dietary vitamin A. Active forms, which are immediately available to the body are obtained from animal products. These are known as retinoids and include retinaldehyde and retinol. Precursors, also known as provitamins, which must be converted to active forms by the body, are obtained from fruits and vegetables containing yellow, orange and dark green pigments, known as carotenoids, the most well-known being β-carotene. For this reason, amounts of vitamin A are measured in Retinol Equivalents (RE). One RE is equivalent to 0.001 mg of retinol, or 0.006 mg of β-carotene, or 3.3 International Units of vitamin A.
In the intestine, vitamin A is protected from being chemically changed by vitamin E. Vitamin A is fat-soluble and can be stored in the body. Most of the vitamin A consumed is stored in the liver. When required by a particular part of the body, the liver releases some vitamin A, which is carried by the blood and delivered to the target cells and tissues.
Dietary intake
The Dietary Reference Intake (DRI) Recommended Daily Amount (RDA) for vitamin A for a 25-year-old male is 900 micrograms/day, or 3000 IU. NHS daily recommended values are slightly lower at 700 micrograms for men and 600 micrograms.[9]
Estimates have changed over time of the rate at which β-carotene is converted to vitamin A in the human body. An early estimate of 6:1 was revised to 12:1 and from recent studies and experimental trials carried out in developing nations it was revised again to 21:1.[3] The implication of the reduced estimate is that larger quantities of β-carotene are needed to yield the necessary dietary requirement of vitamin A. This means that more continents are affected by the deficiency of vitamin A than was previously thought. Changing dietary choices in Africa, Asia, and South America will not be sufficient, and agricultural practices on those continents will need to change.[3]
The Food Standards Agency states that an average adult should not consume more than 1500 micrograms (5000 IU) per day, because this increases the chance of osteoporosis.
During the absorption process in the intestines, retinol is incorporated into chylomicrons as the ester form, and it is these particles that mediate transport to the liver. Liver cells (hepatocytes) store vitamin A as the ester, and when retinol is needed in other tissues, it is de-esterifed and released into the blood as the alcohol. Retinol then attaches to a serum carrier, retinol binding protein, for transport to target tissues. A binding protein inside cells, cellular retinoic acid binding protein, serves to store and move retinoic acid intracellularly. Carotenoid bioavailability ranges between 1/5 to 1/10 of retinol's. Carotenoids are better absorbed when ingested as part of a fatty meal. Also, the carotenoids in vegetables, especially those with tough cell walls (e.g. carrots), are better absorbed when these cell walls are broken up by cooking or mincing.
Deficiency
Vitamin A deficiency is common in developing countries but rarely seen in developed countries. Approximately 250,000 to 500,000 malnourished children in the developing world go blind each year from a deficiency of vitamin A. Night blindness is one of the first signs of vitamin A deficiency. Vitamin A deficiency contributes to blindness by making the cornea very dry and damaging the retina and cornea.
Interventions/remedies
Interventions or remedies in vitamin A deficiency in a deficient population may be enforced using three approaches:(A) through dietary modification involving the adjustment of menu choices of affected persons from available food sources to optimize vitamin A content, (B) enriching commonly eaten and affordable foods with vitamin A, a process called fortification. It involves addition of synthetic vitamin A to staple foods like margarine, bread, flours, cereals and other infant formulae during processing and (C) giving high-doses of vitamin A to the targeted deficient population, a method known as supplementation.[10] Caution should however be exercised when using supplementation as a method of replenishing vitamin A in the body so that upper harmful limits are not attained.
Retinoid overdose (toxicity)
- see Hypervitaminosis A for details
The Tolerable Upper Intake Level (UL) for vitamin A, for a 25-year-old male, is 3,000 micrograms/day, or about 10,000 IU.
Too much vitamin A in retinoid form can be harmful or fatal, resulting in what is known as hypervitaminosis A. The body converts the dimerized form, carotene, into vitamin A as it is needed, therefore high levels of carotene are not toxic compared to the ester (animal) forms. The livers of certain animals, especially those adapted to polar environments, often contain amounts of vitamin A that would be toxic to humans. Thus, vitamin A toxicity is typically reported in Arctic explorers and people taking large doses of synthetic vitamin A. The first documented death possibly caused by vitamin A poisoning was Xavier Mertz, a Swiss scientist who died in January 1913 on an Antarctic expedition that had lost its food supplies and fell to eating its sled dogs. Mertz may have consumed lethal amounts of vitamin A by eating the dogs' livers.[11]
Vitamin A acute toxicity occurs when an individual ingests vitamin A in large amounts more than the daily recommended value in the threshold of 25,000 IU/kg or more. Often, the individual consumes about 3–4 times the RDA's specification.[1] Toxicity of vitamin A is believed to be associated with the intervention methods used to upgrade vitamin A levels in the body such as food modification, fortification and supplementation, all of which are employed to combat vitamin A deficiency [12] Toxicity is classified into two categories: acute and chronic toxicities. The former occurs few hours or days after ingestion of large amounts of vitamin A accidentally or via inappropriate therapy. The later toxicity (Chronic) takes place when about 4,000 IU/kg or more of vitamin A is consumed for a prolonged period of time. Symptoms associated with both toxicities include nausea, blurred vision, fatigue, weight-loss, menstrual abnormalities etc.[13]
Excess vitamin A has also been suspected to be a contributor to osteoporosis. This seems to happen at much lower doses than those required to induce acute intoxication. Only preformed vitamin A can cause these problems, because the conversion of carotenoids into vitamin A is downregulated when physiological requirements are met. An excessive uptake of carotenoids can, however, cause carotenosis.
Dietary supplementation with β-carotene was interestingly associated with an increase in lung cancer when it was studied in a lung cancer prevention trial in male smokers. In non-smokers, the opposite effect has been noted.
Excess preformed vitamin A during early pregnancy has also been associated with a significant increase in birth defects.[14] These defects may be severe, even life-threatening. Even twice the daily recommended amount can cause severe birth defects.[15] The FDA currently recommends that pregnant women get their vitamin A from foods containing β-carotene and that they should ensure that they consume no more than 5,000 IU of preformed vitamin A (if any) per day. Although vitamin A is necessary for fetal development, most women carry stores of vitamin A in their fat cells, so oversupplementation should be strictly avoided.
A review of all randomized controlled trials in the scientific literature by the Cochrane Collaboration published in JAMA in 2007 found that supplementation with β-carotene or vitamin A increased mortality by 5% and 16%, respectively.[16]
Contrary to earlier observations, recent studies emerging from some developing countries (India, Bangladesh and Indonesia) have strongly suggested that dosing expectant mothers in the population in which vitamin A deficiency is common and maternal mortality is high can greatly reduce the maternal mortality rate.[3] Similarly, dosing newborn infants with 50,000 IU (15 mg) of vitamin A within 2 days of birth, can significantly reduce neonatal mortality.[17][18]
Sources
All sources of vitamin A can provide retinol, but retinoids are found naturally in some foods of animal origin. Each of the following contains at least 0.15 mg of retinoids per 1.75–7 oz (50–198 g):
Synthetic sources
Synthetic retinol is marketed under the following trade names: Acon, Afaxin, Agiolan, Alphalin, Anatola, Aoral, Apexol, Apostavit, Atav, Avibon, Avita, Avitol, Axerol, Dohyfral A, Epiteliol, Nio-A-Let, Prepalin, Testavol, Vaflol, Vi-Alpha, Vitpex, Vogan, and Vogan-Neu.
There are three known routes to retinol that are used industrially, all of which start with β-ionone.[20]
Night vision
Night blindness—the inability to see well in dim light—is associated with a deficiency of vitamin A. At first, the most light sensitive (containing more retinal) protein rhodopsin is influenced. Less pigmented retinal iodopsins (three forms/colors in humans), responsible for color vision and sensing relatively high light intensities (day vision), are less impaired at early stages of the vitamin A deficiency. All these protein-pigment complexes are located in the light-sensing cells in eye's retina.
When stimulated by light, rhodopsin splits into a protein and a cofactor: opsin and all-trans-retinal (a form of vitamin A). The regeneration of active rhodopsin requires opsin and 11-cis-retinal. The regeneration of 11-cis-retinal occurs in vertebrates via a sequence of chemical transformations that constitute "the visual cycle" and which occurs primarily in the retinal pigmented epithelial cells.
Without adequate amounts of retinal, regeneration of rhodopsin is incomplete and night blindness occurs.
Closely related chemicals
- Tretinoin (Tradename: Retin-A)
- Isotretinoin (Tradename: Accutane(US), Roaccutane)
- Retinyl palmitate ("vitamin A palmitate")
Genetically engineered vitamin A enriched rice
Because of the high prevalence of vitamin A deficiency in developing countries, there are efforts to produce genetically modified rice rich in β-carotene. The idea is that this would help poor people, who can not afford a varied diet containing sufficient natural sources of vitamin A, meet their dietary needs. The golden rice project is one such effort, and is already undergoing trials.
References
- 1 2 Gropper, S.S., Smith, J.L. and Groff, J.L. (2009) Advanced Nutrition and Human Metabolism. 5th ed., pp. 373–1182.
- ↑ Semba RD (1999). "Vitamin a as "anti-infective" therapy, 1920–1940". The Journal of Nutrition 129 (4): 783–91. PMID 10203551.
- 1 2 3 4 Sommer A (2008). "Vitamin a deficiency and clinical disease: An historical overview". The Journal of Nutrition 138 (10): 1835–9. PMID 18806089.
- 1 2 Duester, G. (2008). "Retinoic acid synthesis and signaling during early organogenesis". Cell 134 (6): 921–31. doi:10.1016/j.cell.2008.09.002. PMC 2632951. PMID 18805086.
- ↑ Dewick, Paul M. (2009) Medicinal Natural Products, Wiley, ISBN 0470741678.
- ↑ Oren T, Sher JA, Evans T. "Hematopoiesis and retinoids: development and disease". Leukemia & Lymphoma. 2003 Nov; 44(11): 1881-91.and Evans T. "Regulation of hematopoiesis by retinoid signalling". Experimental Hematology. 2005 Sep; 33(9): 1055-61.
- ↑ Garcia-Casal MN, Layrisse M, Solano L, Baron MA, Arguello F, Llover D, Ramirez J, Leets I, Tropper E (Mar 1998). "Vitamin A and beta-carotene can improve nonheme iron absorption from rice, wheat and corn by humans". Journal of Nutrition 128 (3): 646–50.
- ↑ Raifen, R.; Altman, Y.; Zadik, Z. (1996). "Vitamin a levels and growth hormone axis". Hormone research 46 (6): 279–281. doi:10.1159/000185101. PMID 9064277.
- ↑ Vitamins and minerals – Vitamin A – NHS Choices. Nhs.uk (2012-11-26). Retrieved on 2013-09-19.
- ↑ Schultink, W (2002). "Use of under-five mortality rate as an indicator for vitamin a deficiency in a population". The Journal of Nutrition 132 (9 Suppl): 2881S–2883S. PMID 12221264.
- ↑ An account of Mertz's illness, retrieved 20 June 2007.
- ↑ Thompson, J and Manore, M (2005) "Nutrients involved in antioxidant function", Ch. 8, pp. 276–283 in Nutrition: An Applied Approach. Pearson Education Inc. Publishers.
- ↑ Mohsen, S.E., Mckinney, K. and Shanti, M.S. (2008). Vitamin A toxicity. Medscape
- ↑ Challem, Jack (1995) Caution Urged With Vitamin A in Pregnancy: But Beta-Carotene is Safe, The Nutrition Reporter Newsletter.
- ↑ Stone, Brad (6 October 1995) Vitamin A and Birth Defects. fda.gov
- ↑ Bjelakovic, G; Nikolova, D; Gluud, LL; Simonetti, RG; Gluud, C (2007). "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis" (PDF). JAMA: the Journal of the American Medical Association 297 (8): 842–57. doi:10.1001/jama.297.8.842. PMID 17327526.
- ↑ Tielsch, J.M, Rahmathullah, L., Thulsiraj, R.D., Katz, J., Coles, C., Sheeladevi, S., John, R and Prakash, K (2007). "Newborn vitamin a dosing reduces the case fatality but not incidence of common childhood morbidities in South India". The Journal of Nutrition 137 (11): 2470–4. PMID 17951487.
- ↑ Klemm, R.D., Labrique, A.B., Christian, P., Rashid, M., Shamim, A.A., Katz, J., Sommer, A and West, K.P. (2008). "Newborn vitamin a supplementation reduced infant mortality in rural Bangladesh". Pediatrics 122 (1): e242–50. doi:10.1542/peds.2007-3448. PMID 18595969.
- ↑ Brown, J. E. (2002). Vitamins and Your Health. Nutrition Now. 3rd ed., pp. 20–1 to 20–20.
- ↑ Ashford's Dictionary of Industrial Chemicals, 3rd ed., 2011, ISBN 978-0-9522674-3-0, p. 9662
External links
- Jane Higdon, "Vitamin A", Micronutrient Information Center, Linus Pauling Institute, Oregon State University
- A commoningly used ingredient to lighten hyper-pigmentation
- NIH Office of Dietary Supplements – Vitamin A
- Retinol binding protein
- Merck Manual of Diagnosis and Therapy: Vitamin A Deficiency
- WHO publications on Vitamin A Deficiency
- BBC News article on Retinol's use as an anti-wrinkle cream
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||