Hair cell
| Hair cell | |
|---|---|
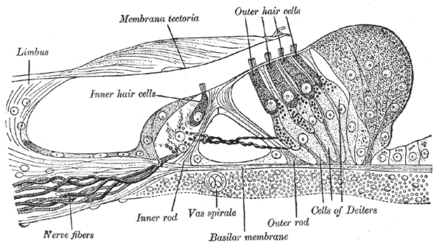 Section through the spiral organ of Corti. Magnified. ("Outer hair cells" labeled near top; "inner hair cells" labeled near center). | |
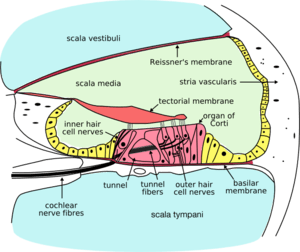 Cross-section of the cochlea. The inner hair cells are located at the termination of the "inner hair cell nerves" and the outer hair cells are located at the termination of the "outer hair cell nerves". | |
| Details | |
| Location | Cochlea |
| Morphology | Unique (see text) |
| Function | Amplify sound waves and transduce auditory information to the Brain Stem |
| Neurotransmitter | Glutamate |
| Presynaptic connections | None |
| Postsynaptic connections | Via auditory nerve to vestibulocochlear nerve to inferior colliculus |
| Identifiers | |
| NeuroLex ID | nifext_61 |
Hair cells are the sensory receptors of both the auditory system and the vestibular system in the ears of all vertebrates. Through mechanotransduction, hair cells detect movement in their environment.[1] In mammals, the auditory hair cells are located within the spiral organ of Corti on the thin basilar membrane in the cochlea of the inner ear. They derive their name from the tufts of stereocilia called hair bundles that protrude from the apical surface of the cell into the fluid-filled cochlear duct. Mammalian cochlear hair cells are of two anatomically and functionally distinct types, known as outer and inner hair cells. Damage to these hair cells results in decreased hearing sensitivity, and because the inner ear hair cells cannot regenerate, this damage is permanent.[2] However, other organisms, such as the frequently studied zebrafish, and birds have hair cells that can regenerate.[3][4]
Hair bundles as sound detectors and amplifiers
Research of the past decades has shown that outer hair cells do not send neural signals to the brain, but that they mechanically amplify low-level sound that enters the cochlea. The amplification may be powered by the movement of their hair bundles, or by an electrically driven motility of their cell bodies. The inner hair cells transform the sound vibrations in the fluids of the cochlea into electrical signals that are then relayed via the auditory nerve to the auditory brainstem and to the auditory cortex.
Results in recent years further indicate that mammals apparently have conserved an evolutionarily earlier type of hair-cell motility. This so-called hair-bundle motility amplifies sound in all non-mammalian land vertebrates. It is affected by the closing mechanism of the mechanical sensory ion channels at the tips of the hair bundles. Thus, the same hair-bundle mechanism that detects sound vibrations also actively "vibrates back" and thereby mechanically amplifies weak incoming sound.
Inner hair cells – from sound to nerve signal

The deflection of the hair-cell stereocilia opens mechanically gated ion channels that allow any small, positively charged ions (primarily potassium and calcium) to enter the cell.[5] Unlike many other electrically active cells, the hair cell itself does not fire an action potential. Instead, the influx of positive ions from the endolymph in the scala media depolarizes the cell, resulting in a receptor potential. This receptor potential opens voltage gated calcium channels; calcium ions then enter the cell and trigger the release of neurotransmitters at the basal end of the cell. The neurotransmitters diffuse across the narrow space between the hair cell and a nerve terminal, where they then bind to receptors and thus trigger action potentials in the nerve. In this way, the mechanical sound signal is converted into an electrical nerve signal. Repolarization of hair cells is done in a special manner. The perilymph in the scala tympani has a very low concentration of positive ions. The electrochemical gradient makes the positive ions flow through channels to the perilymph.
Hair cells chronically leak Ca2+. This leakage causes a tonic release of neurotransmitter to the synapses. It is thought that this tonic release is what allows the hair cells to respond so quickly in response to mechanical stimuli. The quickness of the hair cell response may also be due to that fact that it can increase the amount of neurotransmitter release in response to a change as little as 100 μV in membrane potential.[6]
Outer hair cells – acoustical pre-amplifiers
In mammalian outer hair cells, the receptor potential triggers active vibrations of the cell body. This mechanical response to electrical signals is termed somatic electromotility[7] and drives oscillations in the cell’s length, which occur at the frequency of the incoming sound and provide mechanical feedback amplification. A movie clip showing an isolated outer hair cell moving in response to electrical stimulation can be seen here. Outer hair cells are found only in mammals. While hearing sensitivity of mammals is similar to that of other classes of vertebrates, without functioning outer hair cells, the sensitivity decreases by approximately 50 dB . Outer hair cells extend the hearing range to about 200 kHz in some marine mammals.[8] They have also improved frequency selectivity (frequency discrimination), which is of particular benefit for humans, because it enabled sophisticated speech and music.
The effect of this system is to non-linearly amplify quiet sounds more than large ones so that a wide range of sound pressures can be reduced to a much smaller range of hair displacements.[9] This property of amplification is called the cochlear amplifier.
The molecular biology of hair cells has seen considerable progress in recent years, with the identification of the motor protein (prestin) that underlies somatic electromotility in the outer hair cells. Santos-Sacchi et al. have shown that prestin's function is dependent on chloride channel signaling and that it is compromised by the common marine pesticide tributyltin (TBT). Because this class of pollutant bioconcentrates up the food chain, the effect is pronounced in top marine predators such as orcas and toothed whales.[10]
Neural connection
Neurons of the auditory or vestibulocochlear nerve (the VIIIth cranial nerve) innervate cochlear and vestibular hair cells.[11] The neurotransmitter released by hair cells that stimulates the terminal neurites of peripheral axons of the afferent neurons is thought to be glutamate. At the presynaptic juncture, there is a distinct presynaptic dense body or ribbon. This dense body is surrounded by synaptic vesicles and is thought to aid in the fast release of neurotransmitter.
Nerve fiber innervation is much denser for inner hair cells than for outer hair cells. A single inner hair cell is innervated by numerous nerve fibers, whereas a single nerve fiber innervates many outer hair cells. Inner hair cell nerve fibers are also very heavily myelinated, which is in contrast to the unmyelinated outer hair cell nerve fibers. The region of the basilar membrane supplying the inputs to a particular afferent nerve fibre can be considered to be its receptive field.
Efferent projections from the brain to the cochlea also play a role in the perception of sound. Efferent synapses occur on outer hair cells and on afferent (towards the brain) axons under inner hair cells. The presynaptic terminal bouton is filled with vesicles containing acetylcholine and a neuropeptide called Calcitonin gene-related peptide (CGRP). The effects of these compounds vary, in some hair cells the acetylcholine hyperpolarized the cell, which reduces the sensitivity of the cochlea locally.
Regrowth
Research on the regrowth of cochlear cells may lead to medical treatments that restore hearing. Unlike birds and fish, humans and other mammals are generally incapable of regrowing the cells of the inner ear that convert sound into neural signals when those cells are damaged by age or disease.[4][12] Researchers are making progress toward gene and stem cell therapies that may allow the damaged cells to be regenerated. Because hair cells of auditory and vestibular systems in birds and fish have been found to regenerate, their ability has been studied at length.[4][13] In addition, lateral line hair cells, which have a mechanotransduction function, have been shown to regrow in organisms, such as the zebrafish.[14]
Researchers have identified a mammalian gene that normally acts as a molecular switch to block the regrowth of cochlear hair cells in adults.[15] The Rb1 gene encodes the retinoblastoma protein, which is a tumor suppressor. Rb stops cells from dividing by encouraging their exit from the cell cycle.[16][17] Not only do hair cells in a culture dish regenerate when the Rb1 gene is deleted, but mice bred to be missing the gene grow more hair cells than control mice that have the gene. Additionally, the sonic hedgehog protein has been shown to block activity of the retinoblastoma protein, thereby inducing cell cycle re-entry and the regrowth of new cells.[18]
The cell cycle inhibitor p27kip1 (CDKN1B) has also been found to encourage regrowth of cochlear hair cells in mice following genetic deletion or knock down with siRNA targeting p27.[19][20] Research on hair cell regeneration may bring us closer to clinical treatment for human hearing loss caused by hair cell damage or death.
Additional images
-
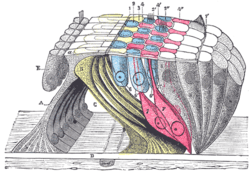
The lamina reticularis and subjacent structures.
-

Inner ear illustration showing semicircular canal, hair cells, ampulla, cupula, vestibular nerve, & fluid
-

Stereocilia of frog inner ear
Notes
- ↑ Lumpkin, Ellen A.; Marshall, Kara L.; Nelson, Aislyn M. (2010). "The cell biology of touch". The Journal of Cell Biology 191 (2): 237–248. doi:10.1083/jcb.201006074.
- ↑ Nadol, Joseph B. (1993). "Hearing loss". New England Journal of Medicine 329 (15): 1092–1102. doi:10.1056/nejm199310073291507.
- ↑ Lush, Mark E.; Piotrowski, Tatjana (2013). "Sensory hair cell regeneration in the zebrafish lateral line". Developmental Dynamics 243 (10): 1187–1202. doi:10.1002/dvdy.24167. PMID 25045019.
- 1 2 3 Cotanche, Douglas A. (1994). "Hair cell regeneration in the bird cochlea following noise damage or ototoxic drug damage". Anatomy and Embryology 189 (1): 1–18. doi:10.1007/bf00193125.
- ↑ Müller, U (October 2008). "Cadherins and mechanotransduction by hair cells". Current opinion in cell biology 20 (5): 557–566. doi:10.1016/j.ceb.2008.06.004. PMC 2692626. PMID 18619539.
- ↑ Chan DK, Hudspeth AJ (February 2005). "Ca2+ current-driven nonlinear amplification by the mammalian cochlea in vitro". Nature Neuroscience 8 (2): 149–155. doi:10.1038/nn1385. PMC 2151387. PMID 15643426.
- ↑ Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y (1985-01-11). "Evoked mechanical responses of isolated cochlear outer hair cells". Science 227 (4683): 194–196. Bibcode:1985Sci...227..194B. doi:10.1126/science.3966153. PMID 3966153.
- ↑ Wartzog D, Ketten DR (1999). "Marine Mammal Sensory Systems". In J. Reynolds, S. Rommel. Biology of Marine Mammals (PDF). Smithsonian Institution Press. p. 132.
- ↑ Hudspeth AJ (2008-08-28). "Making an effort to listen: mechanical amplification in the ear". Neuron 59 (4): 530–45. doi:10.1016/j.neuron.2008.07.012. PMC 2724262. PMID 18760690.
- ↑ Santos-Sacchi Joseph, Song Lei, Zheng Jiefu, Nuttall Alfred L (2006-04-12). "Control of mammalian cochlear amplification by chloride anions". Journal of Neuroscience 26 (15): 3992–8. doi:10.1523/JNEUROSCI.4548-05.2006. PMID 16611815.
- ↑ "Cranial Nerve VIII. Vestibulocochlear Nerve". Meddean. Retrieved 2008-06-04.
- ↑ Edge AS, Chen ZY (2008). "Hair cell regeneration". Current Opinion in Neurobiology 18 (4): 377–82. doi:10.1016/j.conb.2008.10.001. PMID 18929656.
- ↑ Lombarte, Antoni (1993). "Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin". Hearing Research 64 (2): 166–174. doi:10.1016/0378-5955(93)90002-i.
- ↑ Whitfield, T.T (2002). "Zebrafish as a model for hearing and deafness". Journal of Neurobiology 53 (2): 157–171. doi:10.1002/neu.10123.
- ↑ Henderson M (2005-01-15). "Gene that may no longer turn a deaf ear to old age". Times Online.
- ↑ Sage, Cyrille; Huang, Mingqian; Vollrath, Melissa A.; Brown, M. Christian; Hinds, Philip W.; Corey, David P.; Vetter, Douglas E.; Zheng-Yi, Chen (2005). "Essential role of retinoblastoma protein in mammalian hair cell development and hearing". Proceedings of the National Academy of Sciences of the United States of America 103 (19): 7345–7350. doi:10.1073/pnas.0510631103. PMC 1450112. PMID 16648263.
- ↑ Raphael Y, Martin DM (2005). "Deafness: Lack of regulation encourages hair cell growth". Gene Therapy 12 (13): 1021–22. doi:10.1038/sj.gt.3302523.
- ↑ Lu, Na; Chen, Yan; Wang, Zhengmin; Chen, Guoling; Lin, Qin; Chen, Zheng-Yi; Li, Huawei (2013). "Sonic hedgehog initiates cochlear hair cell regeneration through downregulation of retinoblastoma protein". Biochemical and Biophysical Research Communications (Elsevier) 430 (2): 700–705. doi:10.1016/j.bbrc.2012.11.088. PMC 3579567. PMID 23211596.
- ↑ Löwenheim H, Furness DN, Kil J, Zinn C, Gültig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP (1999-03-30). "Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti". Proc Natl Acad Sci U S A 96 (7): 4084–8. doi:10.1073/pnas.96.7.4084. PMC 22424. PMID 10097167. (primary source)
- ↑ Ono K, Nakagawa T, Kojima K, Matsumoto M, Kawauchi T, Hoshino M, Ito J (Dec 2009). "Silencing p27 reverses post-mitotic state of supporting cells in neonatal mouse cochleae". Mol Cell Neurosci 42 (4): 391–8. doi:10.1016/j.mcn.2009.08.011. PMID 19733668. (primary source)
References
- Coffin A, Kelley M, Manley GA, Popper AN. "Evolution of sensory hair cells". pp. 55–94. Missing or empty
|title=(help) in Manley et al. (2004) - Fettiplace R, Hackney CM (2006). "The sensory and motor roles of auditory hair cells". Nature Reviews. Neuroscience 7 (1): 19–29. doi:10.1038/nrn1828. PMID 16371947.
- Kandel ER, Schwartz JH, Jessell TM (2000). Principles of Neural Science (4th ed.). New York: McGraw-Hill. pp. 590–594. ISBN 0-8385-7701-6.
- Manley GA, Popper AN, Fay RR (2004). Evolution of the Vertebrate Auditory System. New York: Springer-Verlag. ISBN 0-387-21093-8.
- Manley GA. "Advances and perspectives in the study of the evolution of the vertebrate auditory system". pp. 360–368. Missing or empty
|title=(help) in Manley et al. (2004) - Rabbitt RD, Boyle R, Highstein SM (1–5 February 2010). "Mechanical amplification by hair cells in the semicircular canals". Proceedings of the National Academy of Sciences 107 (8): 3864–9. doi:10.1073/pnas0906765107. PMC 2840494. PMID 20133682. Lay summary.
- Breneman KD, Brownell WE, Rabbitt RD (22 April 2009). Brezina, Vladimir, ed. "Hair cell bundles: flexoelectric motors of the inner ear". PLOS ONE 4 (4): e5201. Bibcode:2009PLoSO...4.5201B. doi:10.1371/journal.pone.0005201. PMC 2668172. PMID 19384413. Lay summary.
External links
- Molecular Basis of Hearing
- Outer hair cell dancing "rock around the clock"
- Dancing OHC video Yale Ear Lab
- NIF Search - Hair Cell via the Neuroscience Information Framework
- Hair-Tuning-Sound-Sensor A concise report on the recent development of sound sensors based on hair tuning by students of SMMEE, IIT Ropar
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||