Reactive oxygen species


Reactive oxygen species (ROS) are chemically reactive molecules containing oxygen. Examples include peroxides, superoxide, hydroxyl radical, and singlet oxygen.
In a biological context, ROS are formed as a natural byproduct of the normal metabolism of oxygen and have important roles in cell signaling and homeostasis.[1] However, during times of environmental stress (e.g., UV or heat exposure), ROS levels can increase dramatically.[1] This may result in significant damage to cell structures. Cumulatively, this is known as oxidative stress. ROS are also generated by exogenous sources such as ionizing radiation.[2]
Formation and decomposition
The reduction of molecular oxygen (O2) produces superoxide (·O2−) and is the precursor of most other reactive oxygen species:[3]
- O2 + e− → ·O2−
Dismutation of superoxide produces hydrogen peroxide (H2O2):[3]
- 2H+ + ·O2− + ·O2− → H2O2 + O2
Hydrogen peroxide in turn may be partially reduced to hydroxyl radical (·OH) or fully reduced to water:[3]
- H2O2 → HO · + ·OH
- H2O2 + H2O2 → H2O + H2O + O2
Exogenous ROS
Exogenous ROS can be produced from pollutants, tobacco, smoke, drugs, xenobiotics, or radiation.
Ionizing radiation can generate damaging intermediates through the interaction with water, a process termed radiolysis. Since water comprises 55–60% of the human body, the probability of radiolysis is quite high under the presence of ionizing radiation. In the process, water loses an electron and becomes highly reactive. Then through a three-step chain reaction, water is sequentially converted to hydroxyl radical (·OH), hydrogen peroxide (H2O2), superoxide radical (·O2−) and ultimately oxygen (O2).
The hydroxyl radical is extremely reactive that immediately removes electrons from any molecule in its path, turning that molecule into a free radical and so propagating a chain reaction. However, hydrogen peroxide is actually more damaging to DNA than the hydroxyl radical, since the lower reactivity of hydrogen peroxide provides enough time for the molecule to travel into the nucleus of the cell, subsequently wreaking havoc on macromolecules such as DNA.
Endogenous ROS
ROS are produced intracellularly through multiple mechanisms and depending on the cell and tissue types, the major sources being the "professional" producers of ROS NADPH oxidase (NOX) complexes (7 distinct isoforms) in cell membranes, mitochondria, peroxisomes, and endoplasmic reticulum.[4][5] Mitochondria convert energy for the cell into a usable form, adenosine triphosphate (ATP). The process in which ATP is produced, called oxidative phosphorylation, involves the transport of protons (hydrogen ions) across the inner mitochondrial membrane by means of the electron transport chain. In the electron transport chain, electrons are passed through a series of proteins via oxidation-reduction reactions, with each acceptor protein along the chain having a greater reduction potential than the previous. The last destination for an electron along this chain is an oxygen molecule. In normal conditions, the oxygen is reduced to produce water; however, in about 0.1–2% of electrons passing through the chain (this number derives from studies in isolated mitochondria, though the exact rate in live organisms is yet to be fully agreed upon), oxygen is instead prematurely and incompletely reduced to give the superoxide radical (·O2−), most well documented for Complex I and Complex III.[6] Superoxide is not particularly reactive by itself, but can inactivate specific enzymes or initiate lipid peroxidation in its protonated form, hydroperoxyl HO2·. The pKa of hydroperoxyl is 4.8. Thus, at physiological pH, the majority will exist as superoxide anion.
If too much damage is present in mitochondria, a cell undergoes apoptosis or programmed cell death. Bcl-2 proteins are layered on the surface of the mitochondria, detect damage, and activate a class of proteins called Bax, which punch holes in the mitochondrial membrane, causing cytochrome C to leak out. This cytochrome C binds to Apaf-1, or apoptotic protease activating factor-1, which is free-floating in the cell's cytoplasm. Using energy from the ATPs in the mitochondrion, the Apaf-1 and cytochrome C bind together to form apoptosomes. The apoptosomes bind to and activate caspase-9, another free-floating protein. The caspase-9 then cleaves the proteins of the mitochondrial membrane, causing it to break down and start a chain reaction of protein denaturation and eventually phagocytosis of the cell.
Superoxide dismutase
Superoxide dismutases (SOD) are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen. In mammals and most chordates, three forms of superoxide dismutase are present. SOD1 is located primarily in the cytoplasm, SOD2 in the mitochondria and SOD3 is extracellular. The first is a dimer (consists of two units), while the others are tetramers (four subunits). SOD1 and SOD3 contain copper and zinc ions, while SOD2 has a manganese ion in its reactive centre. The genes are located on chromosomes 21, 6, and 4, respectively (21q22.1, 6q25.3 and 4p15.3-p15.1).
The SOD-catalysed dismutation of superoxide may be written with the following half-reactions :
- M(n+1)+ − SOD + O2− → Mn+ − SOD + O2
- Mn+ − SOD + O2− + 2H+ → M(n+1)+ − SOD + H2O2.
where M = Cu (n=1) ; Mn (n=2) ; Fe (n=2) ; Ni (n=2).
In this reaction the oxidation state of the metal cation oscillates between n and n+1.
Catalase, which is concentrated in peroxisomes located next to mitochondria, reacts with the hydrogen peroxide to catalyze the formation of water and oxygen. Glutathione peroxidase reduces hydrogen peroxide by transferring the energy of the reactive peroxides to a very small sulfur-containing protein called glutathione. The sulfur contained in these enzymes acts as the reactive center, carrying reactive electrons from the peroxide to the glutathione. Peroxiredoxins also degrade H2O2, within the mitochondria, cytosol, and nucleus.
- 2 H2O2 → 2 H2O + O2 (catalase)
- 2GSH + H2O2 → GS–SG + 2H2O (glutathione peroxidase)
Singlet oxygen
Another type of reactive oxygen species is singlet oxygen (1O2) which is produced for example as byproduct of photosynthesis in plants. In the presence light and oxygen, photosensitizers such as chlorophyll may convert triplet (3O2) to singlet oxygen:[7]
![^3O_2 \xrightarrow[photosensitizer]{light} ^1O_2](../I/m/d76e93347e9d2cb7d73d0fd343e5c10d.png)
Singlet oxygen is highly reactive, especially with organic compounds that contain double bonds. The resulting damage caused by singlet oxygen reduces the photosynthetic efficiency of chloroplasts. In plants exposed to excess light, the increased production of singlet oxygen can result in cell death.[7] Various substances such as carotenoids, tocopherols and plastoquinones contained in chloroplasts quench singlet oxygen and protect against its toxic effects. In addition to direct toxicity, singlet oxygen acts a signaling molecule.[7] Oxidized products of β-carotene arising from the presence of singlet oxygen act as second messengers that can either protect against singlet oxygen induced toxicity or initiate program cell death. Levels of jasmonate play a key role in the decision between cell acclimation or cell death in response to elevated levels of this reactive oxygen species.[7]
Damaging effects
Effects of ROS on cell metabolism are well documented in a variety of species. These include not only roles in apoptosis (programmed cell death) but also positive effects such as the induction of host defence[8][9]genes and mobilization of ion transport systems. This implicates them in control of cellular function. In particular, platelets involved in wound repair and blood homeostasis release ROS to recruit additional platelets to sites of injury. These also provide a link to the adaptive immune system via the recruitment of leukocytes.
Reactive oxygen species are implicated in cellular activity to a variety of inflammatory responses including cardiovascular disease. They may also be involved in hearing impairment via cochlear damage induced by elevated sound levels, in ototoxicity of drugs such as cisplatin, and in congenital deafness in both animals and humans. ROS are also implicated in mediation of apoptosis or programmed cell death and ischaemic injury. Specific examples include stroke and heart attack.
In general, harmful effects of reactive oxygen species on the cell are most often:[10]
- damage of DNA
- oxidations of polyunsaturated fatty acids in lipids (lipid peroxidation)
- oxidations of amino acids in proteins
- oxidatively deactivate specific enzymes by oxidation of co-factors
Pathogen response
When a plant recognizes an attacking pathogen, one of the first induced reactions is to rapidly produce superoxide (O−
2) or hydrogen peroxide (H
2O
2) to strengthen the cell wall. This prevents the spread of the pathogen to other parts of the plant, essentially forming a net around the pathogen to restrict movement and reproduction.
In the mammalian host, ROS is induced as an antimicrobial defense. To highlight the importance of this defense, individuals with chronic granulomatous disease who have deficiencies in generating ROS, are highly susceptible to infection by a broad range of microbes including Salmonella enterica, Staphylococcus aureus, Serratia marcescens, and Aspergillus spp.
The exact manner in which ROS defends the host from invading microbe is not fully understood. One of the more likely modes of defense is damage to microbial DNA. Studies using Salmonella demonstrated that DNA repair mechanisms were required to resist killing by ROS. More recently, a role for ROS in antiviral defense mechanisms has been demonstrated via Rig-like helicase-1 and mitochondrial antiviral signaling protein. Increased levels of ROS potentiate signaling through this mitochondria-associated antiviral receptor to activate interferon regulatory factor (IRF)-3, IRF-7, and nuclear factor kappa B (NF-κB), resulting in an antiviral state.[11] Respiratory epithelial cells were recently demonstrated to induce mitrochondrial ROS in response to influenza infection. This induction of ROS led to the induction of type III interferon and the induction of an antiviral state, limiting viral replication.[12] In host defense against mycobacteria, ROS play a role, although direct killing is likely not the key mechanism; rather, ROS likely affect ROS-dependent signalling controls, such as cytokine production, autophagy, and granuloma formation.[13]
Oxidative damage
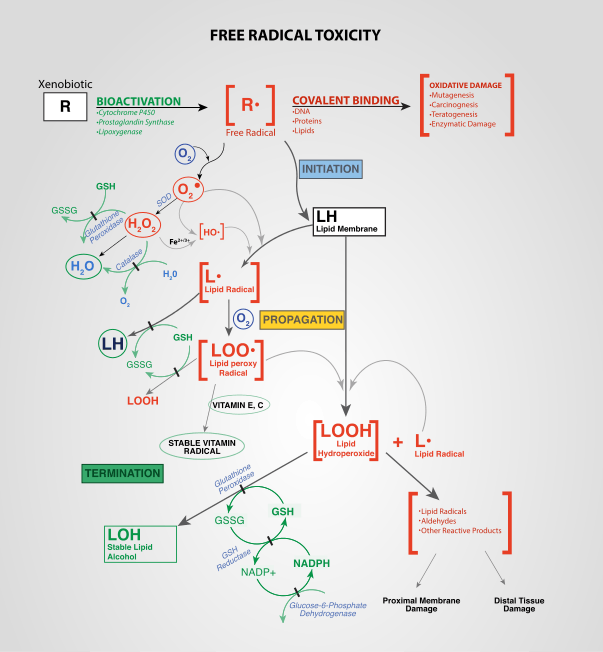
In aerobic organisms the energy needed to fuel biological functions is produced in the mitochondria via the electron transport chain. In addition to energy, reactive oxygen species (ROS) with the potential to cause cellular damage are produced. ROS can damage DNA, RNA, and proteins, which, in theory, contributes to the physiology of ageing.
ROS are produced as a normal product of cellular metabolism. In particular, one major contributor to oxidative damage is hydrogen peroxide (H2O2), which is converted from superoxide that leaks from the mitochondria. Catalase and superoxide dismutase ameliorate the damaging effects of hydrogen peroxide and superoxide, respectively, by converting these compounds into oxygen and hydrogen peroxide (which is later converted to water), resulting in the production of benign molecules. However, this conversion is not 100% efficient, and residual peroxides persist in the cell. While ROS are produced as a product of normal cellular functioning, excessive amounts can cause deleterious effects.[14] Memory capabilities decline with age, evident in human degenerative diseases such as Alzheimer's disease, which is accompanied by an accumulation of oxidative damage. Current studies demonstrate that the accumulation of ROS can decrease an organism's fitness because oxidative damage is a contributor to senescence. In particular, the accumulation of oxidative damage may lead to cognitive dysfunction, as demonstrated in a study in which old rats were given mitochondrial metabolites and then given cognitive tests. Results showed that the rats performed better after receiving the metabolites, suggesting that the metabolites reduced oxidative damage and improved mitochondrial function.[15] Accumulating oxidative damage can then affect the efficiency of mitochondria and further increase the rate of ROS production.[16] The accumulation of oxidative damage and its implications for aging depends on the particular tissue type where the damage is occurring. Additional experimental results suggest that oxidative damage is responsible for age-related decline in brain functioning. Older gerbils were found to have higher levels of oxidized protein in comparison to younger gerbils. Treatment of old and young mice with a spin trapping compound caused a decrease in the level of oxidized proteins in older gerbils but did not have an effect on younger gerbils. In addition, older gerbils performed cognitive tasks better during treatment but ceased functional capacity when treatment was discontinued, causing oxidized protein levels to increase. This led researchers to conclude that oxidation of cellular proteins is potentially important for brain function.[17]
Cause of aging
According to the Free-radical theory, oxidative damage initiated by reactive oxygen species is a major contributor to the functional decline that is characteristic of aging. While studies in invertebrate models indicate that animals genetically engineered to lack specific antioxidant enzymes (such as SOD), in general, show a shortened lifespan (as one would expect from the theory), the converse manipulation, increasing the levels of antioxidant enzymes, has yielded inconsistent effects on lifespan (though some studies in Drosophila do show that lifespan can be increased by the overexpression of MnSOD or glutathione biosynthesizing enzymes). Also contrary to this theory, deletion of mitochondrial SOD2 can extend lifespan in Caenorhabditis elegans.[18]
In mice, the story is somewhat similar. Deleting antioxidant enzymes, in general, yields shorter lifespan, though overexpression studies have not (with some recent exceptions) consistently extended lifespan.[19]
Cancer
ROS are constantly generated and eliminated in the biological system and are required to drive regulatory pathways.[20] Under normal physiologic conditions, cells control ROS levels by balancing the generation of ROS with their elimination by scavenging system. But under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids and DNA, leading to fatal lesions in cell that contribute to carcinogenesis.
Cancer cells exhibit greater ROS stress than normal cells do, partly due to oncogenic stimulation, increased metabolic activity and mitochondrial malfunction. ROS is a double-edged sword. On one hand, at low levels, ROS facilitates cancer cell survival since cell-cycle progression driven by growth factors and receptor tyrosine kinases (RTK) require ROS for activation[21] and chronic inflammation, a major mediator of cancer, is regulated by ROS. On the other hand, a high level of ROS can suppress tumor growth through the sustained activation of cell-cycle inhibitor[22][23] and induction of cell death as well as senescence by damaging macromolecules. In fact, most of the chemotherapeutic and radiotherapeutic agents kill cancer cells by augmenting ROS stress.[24][25] The ability of cancer cells to distinguish between ROS as a survival or apoptotic signal is controlled by the dosage, duration, type, and site of ROS production. Modest levels of ROS are required for cancer cells to survive, whereas excessive levels kill them.
Metabolic adaptation in tumours balances the cells' need for energy with equally important need for macromolecular building blocks and tighter control of redox balance. As a result, production of NADPH is greatly enhanced, which functions as a cofactor to provide reducing power in many enzymatic reactions for macromolecular biosynthesis and at the same time rescuing the cells from excessive ROS produced during rapid proliferation. Cells counterbalance the detrimental effects of ROS by producing antioxidant molecules, such as reduced glutathione (GSH) and thioredoxin (TRX), which rely on the reducing power of NADPH to maintain their activities.[26]
Most risk factors associated with cancer interact with cells through the generation of ROS. ROS then activate various transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), activator protein-1 (AP-1), hypoxia-inducible factor-1α and signal transducer and activator of transcription 3 (STAT3), leading to expression of proteins that control inflammation; cellular transformation; tumor cell survival; tumor cell proliferation; and invasion, agiogenesis as well as metastasis. And ROS also control the expression of various tumor suppressor genes such as p53, retinoblastoma gene (Rb), and phosphatase and tensin homolog (PTEN).[27]
Carcinogenesis
ROS-related oxidation of DNA is one of the main causes of mutations, which can produce several types of DNA damage, including non-bulky (8-oxoguanine and formamidopyrimidine) and bulky (cyclopurine and etheno adducts) base modifications, abasic sites, non-conventional single-strand breaks, protein-DNA adducts, and intra/interstrand DNA crosslinks.[28] It has been estimated that endogenous ROS produced via normal cell metabolism modify approximately 20,000 bases of DNA per day in a single cell. 8-oxoguanine is the most abundant among various oxidized nitrogeneous bases observed. During DNA replication, DNA polymerase mispairs 8-oxoguanine with adenin, leading to a G->T transition mutation. The resulting genomic instability directly contributes to carcinogenesis.
Cell proliferation
Uncontrolled proliferation is a hallmark of cancer cells. Both exogenous and endogenous ROS have been shown to enhance proliferation of cancer cells. The role of ROS in promoting tumor proliferation is further supported by the observation that agents with potential to inhibit ROS generation can also inhibit cancer cell proliferation.[27] Although ROS can promote tumor cell proliferation, a great increase in ROS has been associated with reduced cancer cell proliferation by induction of G2/M cell cycle arrest; increased phosphorylation of ataxia telangiectasia mutated (ATM), checkpoint kinase 1 (Chk 1), Chk 2; and reduced cell division cycle 25 homolog c (CDC25).[29]
Cell death
A cancer cell can die in three ways: apoptosis, necrosis and autophagy. Excessive ROS can induce apoptosis through both the extrinsic and intrinsic pathways.[30] In the extrinsic pathway of apoptosis, ROS are generated by Fas ligand as an upstream event for Fas activation via phosphorylation, which is necessary for subsequent recruitment of Fas-associated protein with death domain and caspase 8 as well as apoptosis induction.[27] In the intrinsic pathway, ROS function to facilitate cytochrome c release by activating pore-stabilizing proteins (Bcl-2 and Bcl-xL) as well as inhibiting pore-destabilizing proteins (Bcl-2-associated X protein, Bcl-2 homologous antagonist/killer).[31] The intrinsic pathway is also known as the caspase cascade and is induced through mitochondrial damage which triggers the release of cytochrome c. DNA damage, oxidative stress, and loss of mitochondrial membrane potential lead to the release of the pro-apoptotic proteins mentioned above stimulating apoptosis.[32] Mitochondrial damage is closely linked to apoptosis and since mitochondria are easily targeted there is potential for cancer therapy.[33]
The cytotoxic nature of ROS is a driving force behind apoptosis, but in even higher amounts, ROS can result in both apoptosis and necrosis, a form of uncontrolled cell death, in cancer cells.[34]
Numerous studies have shown the pathways and associations between ROS levels and apoptosis, but a newer line of study has connected ROS levels and autophagy.[35] ROS can also induce cell death through autophagy, which is a self-catabolic process involving sequestration of cytoplasmic contents (exhausted or damaged organelles and protein aggregates) for degradation in lysosomes.[36] Therefore, autophagy can also regulate the cell’s health in times of oxidative stress. Autophagy can be induced by ROS levels through many different pathways in the cell in an attempt to dispose of harmful organelles and prevent damage, such as carcinogens, without inducing apoptosis.[37] Autophagic cell death can be prompted by the over expression of autophagy where the cell digests too much of itself in an attempt to minimize the damage and can no longer survive. When this type of cell death occurs, an increase or loss of control of autophagy regulating genes is commonly co-observed.[38] Thus, once a more in-depth understanding of autophagic cell death is attained and it’s relation to ROS, this form of programmed cell death may serve as a future cancer therapy. Autophagy and apoptosis are two different cell death mechanisms brought on by high levels of ROS in the cells, however; autophagy and apoptosis rarely act through strictly independent pathways. There is a clear connection between ROS and autophagy and a correlation seen between excessive amounts of ROS leading to apoptosis.[37] The depolarization of the mitochondrial membrane is also characteristic of the initiation of autophagy. When mitochondria are damaged and begin to release ROS, autophagy is initiated to dispose of the damaging organelle. If a drug targets mitochondria and creates ROS, autophagy may dispose of so many mitochondria and other damaged organelles that the cell is no longer viable. The extensive amount of ROS and mitochondrial damage may also signal for apoptosis. The balance of autophagy within the cell and the crosstalk between autophagy and apoptosis mediated by ROS is crucial for a cell’s survival. This crosstalk and connection between autophagy and apoptosis could be a mechanism targeted by cancer therapies or used in combination therapies for highly resistant cancers.
Tumor cell invasion, angiogenesis and metastasis
After growth factor stimulation of RTKs, ROS can trigger activation of signaling pathways involved in cell migration and invasion such as members of the mitogen activated protein kinase (MAPK) family -extracellular regulated kinase (ERK), c-jun NH-2 terminal kinase (JNK) and p38 MAPK. ROS can also promote migration by augmenting phosphorylation of the focal adhesion kinase (FAK) p130Cas and paxilin.[39]
Both in vitro and in vivo, ROS have been shown to induce transcription factors and modulate signaling molecules involved in angiogenesis (MMP, VEGF) and metastasis (upregulation of AP-1, CXCR4, AKT and downregulation of PTEN).[27]
Chronic inflammation and cancer
Experimental and epidemiologic research over the past several years has indicated close associations among ROS, chronic inflammation, and cancer.[27] ROS induces chronic inflammation by the induction of COX-2, inflammatory cytokines (TNFα, interleukin 1 (IL-1), IL-6), chemokines (IL-8, CXCR4) and pro-inflammatory transcription factors (NF-κB).[27] These chemokines and chemokine receptors, in turn, promote invasion and metastasis of various tumor types.
Cancer therapy
Both ROS-elevating and ROS-eliminating strategies have been developed with the former being predominantly used. Cancer cells with elevated ROS levels depend heavily on the antioxidant defense system. ROS-elevating drugs further increase cellular ROS stress level, either by direct ROS-generation (e.g. motexafin gadolinium, elesclomol) or by agents that abrogate the inherent antioxidant system such as SOD inhibitor (e.g. ATN-224, 2-methoxyestradiol) and GSH inhibitor (e.g. PEITC, buthionine sulfoximine (BSO)). The result is an overall increase in endogenous ROS, which when above a cellular tolerability threshold, may induce cell death.[40][41] On the other hand, normal cells appear to have, under lower basal stress and reserve, a higher capacity to cope with additional ROS-generating insults than cancer cells do.[40][42] Therefore, the elevation of ROS in all cells can be used to achieve the selective killing of cancer cells.
Radiotherapy also relies on ROS toxicity to eradicate tumor cells. Radiotherapy uses X-rays, γ-rays as well as heavy particle radiation such as protons and neutrons to induce ROS-mediated cell death and mitotic failure.[27]
Due to the dual role of ROS, both prooxidant and antioxidant-based anticancer agents have been developed. However, modulation of ROS signaling alone seems not to be an ideal approach due to adaptation of cancer cells to ROS stress, redundant pathways for supporting cancer growth and toxicity from ROS-generating anticancer drugs. Combinations of ROS-generating drugs with pharmaceuticals that can break the redox adaptation could be a better strategy for enhancing cancer cell cytotoxicity.[27]
James Watson[43] and others[44] have proposed that lack of intracellular ROS due to a lack of physical exercise may contribute to the malignant progression of cancer, because spikes of ROS are needed to correctly fold proteins in the endoplasmatic reticulum and low ROS levels may thus aspecifically hamper the formation of tumor suppressor proteins.[44] Since physical exercise induces temporary spikes of ROS, this may explain why physical exercise is beneficial for cancer patient prognosis.[45] Moreover, high inducers of ROS such as 2-deoxy-D-glucose and carbohydrate-based inducers of cellular stress induce cancer cell death more potently because they exploit cancer cell high avidity for sugars.[46]
See also
- Antioxidant
- Melanin
- Mitohormesis
- Oxidative stress
- Oxygen toxicity
- Polyphenol antioxidants
- Pro-oxidant
- Reactive nitrogen species
- Iodide
References
- 1 2 Devasagayam T, Tilak JC, Boloor KK, Sane Ketaki S, Ghaskadbi Saroj S, Lele RD (October 2004). "Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects". Journal of Association of Physicians of India (JAPI) 52: 796.
- ↑ Sosa Torres ME, Saucedo-Vázquez JP, Kroneck PM (2015). "Chapter 1, Section 3 The dark side of dioxygen". In Kroneck PM, Torres ME. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences 15. Springer. pp. 1–12. doi:10.1007/978-3-319-12415-5_1.
- 1 2 3 Turrens JF (2003). "Mitochondrial formation of reactive oxygen species". J. Physiol. (Lond.) 552 (Pt 2): 335–44. doi:10.1113/jphysiol.2003.049478. PMC 2343396. PMID 14561818.
- ↑ Muller F (Oct 2000). "The nature and mechanism of superoxide production by the electron transport chain: Its relevance to aging". Journal of the American Aging Association 23 (4): 227–53. doi:10.1007/s11357-000-0022-9. PMID 23604868.
- ↑ Han D, Williams E, Cadenas E (Jan 2001). "Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space". The Biochemical Journal 353 (Pt 2): 411–6. doi:10.1042/0264-6021:3530411. PMC 1221585. PMID 11139407.
- ↑ Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF (February 2013). "Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers". Journal of Hematology & Oncology 6 (19): 19. doi:10.1186/1756-8722-6-19. PMC 3599349. PMID 23442817.
- 1 2 3 4 Laloi C, Havaux M (2015). "Key players of singlet oxygen-induced cell death in plants". Front Plant Sci 6: 39. doi:10.3389/fpls.2015.00039. PMC 4316694. PMID 25699067.
- ↑ Rada B, Leto TL (2008). "Oxidative innate immune defenses by Nox/Duox family NADPH oxidases" (PDF). Contributions to Microbiology. Contributions to Microbiology 15: 164–87. doi:10.1159/000136357. ISBN 978-3-8055-8548-4. PMC 2776633. PMID 18511861. — Review
- ↑ Conner GE, Salathe M, Forteza R (Dec 2002). "Lactoperoxidase and hydrogen peroxide metabolism in the airway". American Journal of Respiratory and Critical Care Medicine 166 (12 Pt 2): S57–61. doi:10.1164/rccm.2206018. PMID 12471090.
- ↑ Brooker RJ (2011). Genetics: analysis and principles (4th ed.). McGraw-Hill Science. ISBN 978-0-07-352528-0.
- ↑ West AP et al 2011 Nature Reviews Immunology 11, 389-402
- ↑ Kim HJ, Kim CH, Ryu JH, Kim MJ, Park CY, Lee JM, Holtzman MJ, Yoon JH (2013). "Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells". American Journal of Respiratory Cell and Molecular Biology 49 (5): 855–65. doi:10.1165/rcmb.2013-0003OC. PMID 23786562.
- ↑ Deffert C, Cachat J, Krause KH (Aug 2014). "Phagocyte NADPH oxidase, chronic granulomatous disease and mycobacterial infections". Cellular Microbiology 16 (8): 1168–78. doi:10.1111/cmi.12322. PMID 24916152.
- ↑ Patel RP, T Cornwell T, Darley-Usmar VM (1999). "The biochemistry of nitric oxide and peroxynitrite: implications for mitochondrial function". In Packer L, Cadenas E. Understanding the process of aging: the roles of mitochondria, free radicals, and antioxidants. New York, N.Y: Marcel Dekker. pp. 39–56. ISBN 0-8247-1723-6.
- ↑ Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN (Feb 2002). "Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha -lipoic acid". Proceedings of the National Academy of Sciences of the United States of America 99 (4): 2356–61. Bibcode:2002PNAS...99.2356L. doi:10.1073/pnas.261709299. PMC 122369. PMID 11854529.
- ↑ Stadtman ER (Aug 1992). "Protein oxidation and aging". Science 257 (5074): 1220–4. Bibcode:1992Sci...257.1220S. doi:10.1126/science.1355616. PMID 1355616.
- ↑ Carney JM, Starke-Reed PE, Oliver CN, Landum RW, Cheng MS, Wu JF, Floyd RA (1991). "Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone". Proceedings of the National Academy of Sciences U.S.A. 88 (9): 3633–6. Bibcode:1991PNAS...88.3633C. doi:10.1073/pnas.88.9.3633. PMC 51506. PMID 1673789.
- ↑ Van Raamsdonk JM, Hekimi S (Feb 2009). "Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans". PLoS Genetics 5 (2): e1000361. doi:10.1371/journal.pgen.1000361. PMC 2628729. PMID 19197346.
- ↑ Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (Aug 2007). "Trends in oxidative aging theories". Free Radical Biology & Medicine 43 (4): 477–503. doi:10.1016/j.freeradbiomed.2007.03.034. PMID 17640558.
- ↑ Dickinson BC, Chang CJ (Aug 2011). "Chemistry and biology of reactive oxygen species in signaling or stress responses". Nature Chemical Biology 7 (8): 504–11. doi:10.1038/nchembio.607. PMC 3390228. PMID 21769097.
- ↑ Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ (Mar 1997). "Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts". Science 275 (5306): 1649–52. doi:10.1126/science.275.5306.1649. PMID 9054359.
- ↑ Ramsey MR, Sharpless NE (Nov 2006). "ROS as a tumour suppressor?". Nature Cell Biology 8 (11): 1213–5. doi:10.1038/ncb1106-1213. PMID 17077852.
- ↑ Takahashi A, Ohtani N, Yamakoshi K, Iida S, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E (Nov 2006). "Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence". Nature Cell Biology 8 (11): 1291–7. doi:10.1038/ncb1491. PMID 17028578.
- ↑ Renschler MF (Sep 2004). "The emerging role of reactive oxygen species in cancer therapy". European Journal of Cancer 40 (13): 1934–40. doi:10.1016/j.ejca.2004.02.031. PMID 15315800.
- ↑ Toler SM, Noe D, Sharma A (2006). "Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme". Neurosurgical Focus 21 (6): E10. doi:10.3171/foc.2006.21.6.1. PMID 17341043.
- ↑ Cairns RA, Harris IS, Mak TW (Feb 2011). "Regulation of cancer cell metabolism". Nature Reviews. Cancer 11 (2): 85–95. doi:10.1038/nrc2981. PMID 21258394.
- 1 2 3 4 5 6 7 8 Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB (Jun 2012). "Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy". Antioxidants & Redox Signaling 16 (11): 1295–322. doi:10.1089/ars.2011.4414. PMC 3324815. PMID 22117137.
- ↑ Waris G, Ahsan H (2006). "Reactive oxygen species: role in the development of cancer and various chronic conditions". Journal of Carcinogenesis 5: 14. doi:10.1186/1477-3163-5-14. PMC 1479806. PMID 16689993.
- ↑ Ames BN (Sep 1983). "Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases". Science 221 (4617): 1256–64. doi:10.1126/science.6351251. PMID 6351251.
- ↑ Ozben T (Sep 2007). "Oxidative stress and apoptosis: impact on cancer therapy". Journal of Pharmaceutical Sciences 96 (9): 2181–96. doi:10.1002/jps.20874. PMID 17593552.
- ↑ Martindale JL, Holbrook NJ (Jul 2002). "Cellular response to oxidative stress: signaling for suicide and survival". Journal of Cellular Physiology 192 (1): 1–15. doi:10.1002/jcp.10119. PMID 12115731.
- ↑ Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007). "Self-eating and self-killing: crosstalk between autophagy and apoptosis". Nat. Rev. Mol. Cell Biol. 8 (9): 741–52. doi:10.1038/nrm2239. PMID 17717517.
- ↑ Fulda S, Galluzzi L, Kroemer G (2010). "Targeting mitochondria for cancer therapy". Nat Rev Drug Discov 9 (6): 447–64. doi:10.1038/nrd3137. PMID 20467424.
- ↑ Hampton MB, Orrenius S (Sep 1997). "Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis". FEBS Letters 414 (3): 552–6. doi:10.1016/s0014-5793(97)01068-5. PMID 9323034.
- ↑ Gibson SB (Oct 2010). "A matter of balance between life and death: targeting reactive oxygen species (ROS)-induced autophagy for cancer therapy". Autophagy 6 (7): 835–7. doi:10.4161/auto.6.7.13335. PMID 20818163.
- ↑ Shrivastava A, Kuzontkoski PM, Groopman JE, Prasad A (Jul 2011). "Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy". Molecular Cancer Therapeutics 10 (7): 1161–72. doi:10.1158/1535-7163.MCT-10-1100. PMID 21566064.
- 1 2 Scherz-Shouval R, Elazar Z (Sep 2007). "ROS, mitochondria and the regulation of autophagy". Trends in Cell Biology 17 (9): 422–7. doi:10.1016/j.tcb.2007.07.009. PMID 17804237.
- ↑ Xie Z, Klionsky DJ (Oct 2007). "Autophagosome formation: core machinery and adaptations". Nature Cell Biology 9 (10): 1102–9. doi:10.1038/ncb1007-1102. PMID 17909521.
- ↑ Tochhawng L, Deng S, Pervaiz S, Yap CT (May 2013). "Redox regulation of cancer cell migration and invasion". Mitochondrion 13 (3): 246–53. doi:10.1016/j.mito.2012.08.002. PMID 22960576.
- 1 2 Kong Q, Beel JA, Lillehei KO (Jul 2000). "A threshold concept for cancer therapy". Medical Hypotheses 55 (1): 29–35. doi:10.1054/mehy.1999.0982. PMID 11021322.
- ↑ Schumacker PT (Sep 2006). "Reactive oxygen species in cancer cells: live by the sword, die by the sword". Cancer Cell 10 (3): 175–6. doi:10.1016/j.ccr.2006.08.015. PMID 16959608.
- ↑ Trachootham D, Alexandre J, Huang P (Jul 2009). "Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?". Nature Reviews. Drug Discovery 8 (7): 579–91. doi:10.1038/nrd2803. PMID 19478820.
- ↑ Watson JD (Mar 2014). "Type 2 diabetes as a redox disease". Lancet 383 (9919): 841–3. doi:10.1016/s0140-6736(13)62365-x. PMID 24581668.
- 1 2 Molenaar RJ, van Noorden CJ (Sep 2014). "Type 2 diabetes and cancer as redox diseases?". Lancet 384 (9946): 853. doi:10.1016/s0140-6736(14)61485-9. PMID 25209484.
- ↑ Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, Baumgartner RN, Baumgartner KB, Bernstein L (Aug 2008). "Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study". Journal of Clinical Oncology 26 (24): 3958–64. doi:10.1200/jco.2007.15.9822. PMID 18711185.
- ↑ Ndombera, Fidelis T.; VanHecke, Garrett C.; Nagi, Shima; Ahn, Young-Hoon. "Carbohydrate-based inducers of cellular stress for targeting cancer cells". Bioorganic & Medicinal Chemistry Letters. doi:10.1016/j.bmcl.2016.01.063.
Further reading
- Sen CK (2003). "The general case for redox control of wound repair". Wound Repair and Regeneration 11 (6): 431–8. doi:10.1046/j.1524-475X.2003.11607.x. PMID 14617282.
- Krötz F, Sohn HY, Gloe T, Zahler S, Riexinger T, Schiele TM, Becker BF, Theisen K, Klauss V, Pohl U (Aug 2002). "NAD(P)H oxidase-dependent platelet superoxide anion release increases platelet recruitment". Blood 100 (3): 917–24. doi:10.1182/blood.V100.3.917. PMID 12130503.
- Pignatelli P, Pulcinelli FM, Lenti L, Gazzaniga PP, Violi F (Jan 1998). "Hydrogen peroxide is involved in collagen-induced platelet activation". Blood 91 (2): 484–90. PMID 9427701.
- Guzik TJ, Korbut R, Adamek-Guzik T (Dec 2003). "Nitric oxide and superoxide in inflammation and immune regulation". Journal of Physiology and Pharmacology 54 (4): 469–87. PMID 14726604.
External links
- Nobel laureate James Watson's novel hypothesis
- Antioxidants don't work, but no one wants to hear it.
- http://ncrc.appstate.edu/research-focus/current-and-upcoming-research
|