Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation. When their existence is indicated, reactive intermediates can help explain how a chemical reaction takes place.[1][2][3][4]
Most chemical reactions take more than one elementary step to complete, and a reactive intermediate is a high-energy, yet stable, product that exists only in one of the intermediate steps. The series of steps together make a reaction mechanism. A reactive intermediate differs from a reactant or product or a simple reaction intermediate only in that it cannot usually be isolated but is sometimes observable only through fast spectroscopic methods. It is stable in the sense that an elementary reaction forms the reactive intermediate and the elementary reaction in the next step is needed to destroy it.
When a reactive intermediate is not observable, its existence must be inferred through experimentation. This usually involves changing reaction conditions such as temperature or concentration and applying the techniques of chemical kinetics, chemical thermodynamics, or spectroscopy. Reactive intermediates based on carbon are radicals, carbenes, carbocations, carbanions, arynes, and carbynes.
- carbon reactive intermediates
-
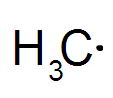
Radical
-

Carbene
-

Carbocation
-

Carbanion
-

Carbyne
-

Benzyne (an aryne)
Common features
Reactive intermediates have several features in common:
- low concentration with respect to reaction substrate and final reaction product
- with the exception of carbanions, these intermediates do not obey the lewis octet rule, hence the high reactivity
- often generated on chemical decomposition of a chemical compound
- it is often possible to prove the existence of this species by spectroscopic means
- cage effects have to be taken into account
- often stabilisation by conjugation or resonance
- often difficult to distinguish from a transition state
- prove existence by means of chemical trapping
Other reactive intermediates
- nitrenes
- Phosphinidenes
- Ion-neutral complex
- carbenoid
- Keto anions
- tetrahedral intermediates in carbonyl addition reactions
- oxocarbenium ions
- Phosphoryl nitride
References
- ↑ Carey, Francis A.; Sundberg, Richard J.; (1984). Advanced Organic Chemistry Part A Structure and Mechanisms (2nd ed.). New York N.Y.: Plenum Press. ISBN 0-306-41198-9.
- ↑ March Jerry; (1885). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. ISBN 0-471-85472-7
- ↑ Gilchrist T.C.;Rees C.W.; (1969) carbenes, nitrenes and arynes. Nelson. London.
- ↑ Reactive intermediate chemistry , Robert A. Moss,Matthew Platz,Maitland Jones