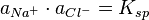Dissolution (chemistry)

The dissolution of gases, liquids, or solids into a liquid or other solvent is a process by which these original states become solutes (dissolved components), forming a solution of the gas, liquid, or solid in the original solvent. Solid solutions, often called salts, are the result of dissolution of one solid into another, and occur, e.g., in metal alloys, where their formation is governed and described by the relevant phase diagram. In the case of a crystalline solid dissolving in a liquid, the crystalline structure must be disintegrated such that the separate atoms, ions, or molecules are released. For liquids and gases, the molecules must be able to form non-covalent intermolecular interactions with those of the solvent for a solution to form.
The free energy of the overall, isolated process of dissolution must be negative for it to occur, where the component free energies contributing include those describing the disintegration of the associations holding the original solute components together, the original associations of the bulk solvent, and the old and new associations between the undissolved and dissolved materials.
Dissolution is of fundamental importance in all chemical processes, natural and unnatural, from the decomposition of a dying organism and return of its chemical constituents into the biosphere, to the laboratory testing of new, man-made soluble drugs, catalysts, etc. Dissolution testing is widely used in industry, including in the pharmaceutical industry to prepare and formulate chemical agents of consistent quality that will dissolve, optimally, in their target millieus as they were designed.
Dissolution by class of compound
Gases
Gaseous elements and compounds will dissolve in liquids dependent on the interaction of their bonds with the liquid solvent.
Liquids
Gaseous elements and compounds may also dissolve in another liquid depending on the compatibility of the chemical and physical bonds in the substance with those of the solvent. Hydrogen bonds play an important role in aqueous dissolution.
Ionic compounds
For ionic compounds, dissolution takes place when the ionic lattice breaks up and the separate ions are then solvated. This most commonly occurs in polar solvents, such as water or ammonia:
- NaCl(s) → Na+(aq) + Cl−(aq)
In a colloidal dispersed system, small dispersed particles of the ionic lattice exist in equilibrium with the saturated solution of the ions, i.e.
- NaCl(aq)
 Na+(aq) + Cl−(aq)
Na+(aq) + Cl−(aq)
The solubility of ionic salts in water is generally determined by the degree of solvation of the ions by water molecules. Such coordination complexes occur by water donating spare electrons on the oxygen atom to the ion. The behavior of this system is characterised by the activity coefficients of the components and the solubility product, defined as:
The ability of an ion to preferentially dissolve (as a result of unequal activities) is classified as the Potential Determining Ion. This in turn results in the remaining particle possessing either a net positive/negative surface charge.
Polar compounds
Polar solid compounds can be amorphous or crystalline. Crystalline solids dissolve with breakdown of their crystal lattice, and due to their polarity, or non-polarity, mix with the solvent.
Polymers
The solubility of polymers depends on the chemical bonds present in the backbone chain and their compatibility with those of the solvent. The Hildebrand solubility parameter is commonly used to evaluate polymer solubility. The closer the value of the parameters, the more likely dissolution will occur.
Rate of dissolution
The rate of dissolution quantifies the speed of the dissolution process. It depends on the chemical natures of the solvent and solute, the temperature (and possibly to a small degree, the pressure), the degree of undersaturation, the presence of a means of mixing during the dissolution, the interfacial surface area, and the presence of "inhibitors" (e.g., substances adsorbed on the surface).
The rate can be often expressed by the Noyes-Whitney Equation or the Nernst and Brunner equation[1] of the form:
where:
- m, mass of dissolved material
- t, time
- A, surface area of the interface between the dissolving substance and the solvent
- D, diffusion coefficient
- d, thickness of the boundary layer of the solvent at the surface of the dissolving substance
- Cs, mass concentration of the substance on the surface
- Cb, mass concentration of the substance in the bulk of the solvent
For dissolution limited by diffusion, Cs is equal to the solubility of the substance. When the dissolution rate of a pure substance is normalized to the surface area of the solid (which usually changes with time during the dissolution process), then it is expressed in kg/m2s and referred to as "intrinsic dissolution rate". The intrinsic dissolution rate is defined by the United States Pharmacopeia.
Dissolution rates vary by orders of magnitude between different systems. Typically, very low dissolution rates parallel low solubilities, and substances with high solubilities exhibit high dissolution rates, as suggested by the Noyes-Whitney equation. However, this is not a rule.
See also
References
- ↑ Aristides Dokoumetzidis, Panos Macheras, 2006, "A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System", Int. J. Pharm. 321(1-2), pp. 1–11, DOI 10.1016/j.ijpharm.2006.07.011, see , accessed 19 June 2015.
Further reading
- Brady, Patrick V.; House, William A. (1996). "4. Surface-controlled dissolution and growth of minerals". In Brady, Patrick V. Physics and chemistry of mineral surfaces. Boca Raton, Fla.: CRC Press. pp. 226–298. ISBN 9780849383519.
- Kumar, Vijai; Tewari, Divya (2006). "Dissolution". In Troy, David B.; Beringer, Paul. Remington: The Science and Practice of Pharmacy. Lippincott Williams & Wilkins. pp. 672–690. ISBN 9780781746731.
- Whitten, Kenneth W. (2014). Chemistry (10th ed.). Belmont, CA: Brooks/Cole Cengage Learning. pp. 506–514. ISBN 9781133610663.
|