Pyrimethamine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
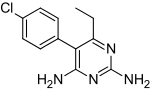
5-(4-chlorophenyl)-6-ethyl- 2,4-pyrimidinediamine | |
| Clinical data | |
| Pronunciation | /ˌpɪrᵻˈmɛθəmin/ |
| Trade names | Daraprim |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601050 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | well-absorbed |
| Protein binding | 87% |
| Metabolism | Hepatic |
| Biological half-life | 96 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
58-14-0 |
| ATC code |
P01BD01 QP51AX51 (combinations) |
| PubChem | CID 4993 |
| IUPHAR/BPS | 4800 |
| DrugBank |
DB00205 |
| ChemSpider |
4819 |
| UNII |
Z3614QOX8W |
| KEGG |
D00488 |
| ChEBI |
CHEBI:8673 |
| ChEMBL |
CHEMBL36 |
| PDB ligand ID | CP6 (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C12H13ClN4 |
| Molar mass | 248.71 g/mol |
| |
| |
| (verify) | |
Pyrimethamine (trade name Daraprim) is a medication used for protozoal infections. It is commonly used as an antimalarial drug (for both treatment and prevention of malaria), and to treat Toxoplasma gondii infections, particularly when combined with the sulfonamide antibiotic sulfadiazine when treating HIV-positive individuals.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[1]
Medical uses
Pyrimethamine is typically given with a sulfonamide and folinic acid:[2]
- Sulfonamides inhibit dihydropteroate synthetase, an enzyme that participates in folic acid synthesis from para-aminobenzoic acid. Hence, sulfonamides work synergistically with pyrimethamine by blocking a different enzyme needed for folic acid synthesis.
- Folinic acid (leucovorin) is a folic acid derivative converted to tetrahydrofolate, the primary active form of folic acid, in vivo without relying on dihydrofolate reductase. Folinic acid reduces side effects related to folate deficiency in the patient.
It is primarily active against Plasmodium falciparum, but also against Plasmodium vivax.[3] Due to the emergence of pyrimethamine-resistant strains of P. falciparum, pyrimethamine alone is seldom used now. In combination with a long-acting sulfonamide such as sulfadiazine, it is widely used, such as in Fansidar though resistance to this combination is increasing.[3] It has been used in the treatment of actinomycosis and isosporiasis, and for the treatment and prevention of Pneumocystis jirovecii pneumonia.
In 2011, researchers discovered that pyrimethamine can increase β-hexosaminidase activity, thus potentially slowing down the progression of late-onset Tay–Sachs disease.[4] It is being evaluated in clinical trials as a treatment for amyotrophic lateral sclerosis.[5]
Mechanism of resistance
Resistance to pyrimethamine is widespread. Mutations in the malarial gene for dihydrofolate reductase may reduce its effectiveness.[6] These mutations decrease the binding affinity between pyrimethamine and dihydrofolate reductase via loss of hydrogen bonds and steric interactions.[7]
Side effects
Pyrimethamine can cause a rash, and if higher doses are used (such as for toxoplasmosis), it can cause gastrointestinal symptoms such as nausea, vomiting, abdominal cramps, dry mouth, weight loss, and diarrhea. Central nervous system effects include headache, ataxia, and rarely seizures and haematologic side effects such as leucopenia and anaemia.[2]
Contraindications
Pyrimethamine is contraindicated in patients with:[2]
- Folate-deficiency anaemia
- Epilepsy
- Pregnancy, especially during the first trimester, due to the possible detrimental effects an antifolate such as pyrimethamine might have on organogenesis
Interactions
Other antifolate agents such as methotrexate and trimethoprim may potentiate the antifolate actions of pyrimethamine, leading to potential folate deficiency, anaemia, and other blood dyscrasias.[2]
Physicochemistry
It is a white, colourless, crystalline powder; it is practically insoluble in water and slightly soluble in ethanol, chloroform, and acetone.[3] It is unstable in the presence of air and light.[3] It is chemically a diaminopyrimidine derivative.[3]
Mechanism of action
Pyrimethamine interferes with tetrahydrofolic acid synthesis from folic acid by inhibiting the enzyme dihydrofolate reductase (DHFR).[8] Tetrahydrofolic acid is needed for DNA and RNA synthesis in many species, including protozoa.[8] It has also been found to reduce the expression of SOD1, a key protein involved in amyotrophic lateral sclerosis.[9][10]
History
The Nobel Prize-winning American scientist Gertrude Elion developed the drug at Burroughs-Wellcome (now part of GlaxoSmithKline) to combat malaria.[11] Pyrimethamine has been available since 1953,[12] and is not subject to any unexpired patent.[13] However, in the United States, the market for this product is sufficiently small that no generic manufacturer has emerged. In 2010, GlaxoSmithKline sold the marketing rights for Daraprim to CorePharma. Impax Laboratories sought to buy CorePharma in 2014, and completed the acquisition, including Daraprim, in March 2015.[14] In August 2015, the rights were bought by Turing Pharmaceuticals.[15] Turing subsequently became known for a price hike controversy when it raised the price of a dose of the drug in the U.S. market from US$13.50 to US$750, a 5,500% increase.[16]
Availability and price
In the United States, as of 2015, with Turing Pharmaceuticals' acquisition of the US marketing rights for Daraprim tablets,[17] Daraprim has become a single-source and specialty pharmacy item, and the price of Daraprim has been increased.[18] The cost of a monthly course for a person on 75 mg dose rose to about $75,000/month, or $750 per tablet.[19][20] Outpatients can no longer obtain Daraprim from their community pharmacy, but only through a single dispensing pharmacy, Walgreens Specialty Pharmacy, and institutions can no longer order from their general wholesaler, but have to set up an account with the Daraprim Direct program.[18][21] Presentations from Retrophin, a company formerly headed by Martin Shkreli, CEO of Turing, from which Turing acquired the rights to Daraprim, suggest that a closed distribution system could prevent generic competitors from legally obtaining the drugs for the bioequivalence studies required for FDA approval of a generic drug.[21]
Martin Shkreli, CEO of Turing, defended the price hike by saying, "If there was a company that was selling an Aston Martin at the price of a bicycle, and we buy that company and we ask to charge Toyota prices, I don't think that that should be a crime."[22][23] As a result of the backlash, Shkreli hired a crisis public relations firm to help explain his fund's move.[24] Turing Pharmaceuticals announced on November 24, 2015 "that it would not reduce the list price of that drug after all" but they will offer various patient assistance programs.[25] However, New York Times journalist Andrew Pollack noted that these programs "are standard for companies selling extremely high-priced drugs. They enable the patients to get the drug while pushing most of the costs onto insurance companies and taxpayers."[25]
On December 17, 2015, Shkrelli was arrested by federal agents on securities fraud related to a hedge fund firm he founded. "The federal case against him has nothing to do with pharmaceutical costs, however. Prosecutors in Brooklyn charged him with illegally taking stock from Retrophin Inc., a biotechnology firm he started in 2011, and using it to pay off debts from unrelated business dealings. He was later ousted from the company, where he’d been chief executive officer, and sued by its board. In the case that closely tracks that suit, federal prosecutors accused Shkreli of engaging in a complicated shell game after his defunct hedge fund, MSMB Capital Management, lost millions. He is alleged to have made secret payoffs and set up sham consulting arrangements."[26] He resigned as CEO of Turing Pharmaceuticals the next day.[27]
The price increase has been fiercely criticised by physician groups such as HIV Medicine Associates and Infectious Diseases Society of America.[28]
In India, over a dozen pharmaceutical companies manufacture and sell pyrimethamine tablets and, multiple combinations of generic pyrimethamine are available for a price ranging from US$0.04–$0.10 each (3–7 rupees).[29][30][31][32]
In the UK, the same drug is available from GSK at a cost of US$20 (£13) for 30 tablets (approximately $0.66 each).[33]
In Australia, the drug is available in most pharmacists at a cost of US$9.35 (A$12.99) for 50 tablets (approximately US$0.18 each).[34]
In Brazil, the drug is available for R$0.07 a pill, or about US$0.02.[35]
In Canada, the drug was reportedly discontinued in 2013 but hospitals may make the drug in-house when it is needed.[36] As of December 2015, Daraprim imported into Canada directly from GSK UK is available from an online pharmacy for US$2.20 per tablet.[37]
On October 22, 2015, Imprimis Pharmaceuticals announced it has made available compounded and customizable formulations of pyrimethamine and leucovorin in oral capsules starting as low as $99.00 for a 100 count bottle in the United States.[38]
References
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 3 4 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- 1 2 3 4 5 Brayfield, A, ed. (13 December 2013). "Pyrimethamine". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 12 April 2014.
- ↑ Osher, E; Fattal-Valevski, A; Sagie, L; Urshanski, N; Amir-Levi, Y; Katzburg, S; Peleg, L; Lerman-Sagie, T; Zimran, A; Elstein, D; Navon, R; Stern, N; Valevski, A (March 2011). "Pyrimethamine increases β-hexosaminidase A activity in patients with Late Onset Tay Sachs.". Molecular Genetics and Metabolism 102 (3): 356–63. doi:10.1016/j.ymgme.2010.11.163. PMID 21185210.
- ↑ "Pyrimethamine ALS trial".
- ↑ Gatton M.L.; et al. (2004). "Evolution of resistance to sulfadoxine-pyrimethamine in Plasmodium falciparum". Antimicrob Agents Chemother 48 (6): 2116–23. doi:10.1128/AAC.48.6.2116-2123.2004. PMC 415611. PMID 15155209.
- ↑ Sirichaiwat C, et al. (2004). "Target guided synthesis of 5-benzyl-2,4-diamonopyrimidines: their antimalarial activities and binding affinities to wild type and mutant dihydrofolate reductases from Plasmodium falciparum". J Med Chem 47 (2): 345–54. doi:10.1021/jm0303352. PMID 14711307.
- 1 2 "PRODUCT INFORMATION DARAPRIM TABLETS". TGA eBusiness Services. Aspen Pharmacare Australia Pty Ltd. 5 December 2011. p. 1. Retrieved 12 April 2014.
- ↑ Limpert, AS; Mattmann, ME; Cosford, ND (2013). "Recent progress in the discovery of small molecules for the treatment of amyotrophic lateral sclerosis (ALS)." (PDF). Beilstein Journal of Organic Chemistry 9: 717–32. doi:10.3762/bjoc.9.82. PMC 3678841. PMID 23766784.
- ↑ Lange, DJ; Andersen, PM; Remanan, R; Marklund, S; Benjamin, D (April 2013). "Pyrimethamine decreases levels of SOD1 in leukocytes and cerebrospinal fluid of ALS patients: a phase I pilot study.". Amyotrophic Lateral Sclerosis & Frontotemporal Degeneration 14 (3): 199–204. doi:10.3109/17482968.2012.724074. PMID 22985433.
- ↑ Vasudevan, D.M.; Sreekumari, S.; Vaidyanathan, Kannan (2013). Textbook of Biochemistry for Medical Students. JP Medical Ltd. p. 491. ISBN 9789350905302. OCLC 843532694. Retrieved January 15, 2016.
- ↑ Ariana Eunjung Cha (2015-09-22). "CEO who raised price of old pill more than $700 calls journalist a 'moron' for asking why". The Washington Post.
- ↑ FDA AccessData entry for Pyrimethamine, accessed September 24, 2015
- ↑ Pollack, Andrew (20 September 2015). "Drug Goes From $13.50 a Tablet to $750, Overnight". New York Times. Retrieved 17 December 2015.
- ↑ John LaMattina (2015-09-21). "Here's A Way For Pharma To Prevent Outrageous Generic Price Increases -- And Help Its Reputation". Forbes.
- ↑ Kliff, Sarah (September 22, 2015). "Vox Explainers: A Drug Company Raised a Pill's Price 5,500 Percent Because, in America, It Can". Vox (online). Retrieved December 9, 2015.
- ↑ Turing Pharmaceuticals AG Turing Pharmaceuticals AG Acquires U.S. Marketing Rights to DARAPRIM® (pyrimethamine) 10 August 2015, PR Newswire Association LLC
- 1 2 Monica V. Mahoney New Pyrimethamine Dispensing Program: What Pharmacists Should Know PharmacyTimes, July 17, 2015
- ↑ ANDREW POLLACK (20 September 2015). "Drug Goes From $13.50 a Tablet to $750, Overnight". Retrieved 21 September 2015.
- ↑ "WATCH: Ex-hedge funder who hiked AIDS pill cost by 5,500 percent says drug 'still underpriced'". RawStory.com. Retrieved 22 September 2015.
- 1 2 "The Most Unconscionable Drug Price Hike I Have Yet Seen", by Derek Lowe, September 11, 2014, In the Pipeline.
- ↑ Ramsey, Lydia (22 Sep 2015). "A pharma CEO tried to defend his decision to jack up the price of a critical drug by 5,000% — and it backfired". Business Insider.
- ↑ Reuters (22 Sep 2015). "Company hikes price of popular drug". Reuters.
- ↑ Tannahill, Jason (9 Oct 2015). "PR Man Allan Ripp Representing The “Most Hated Man in America”". EverythingPR.
- 1 2 Pollack, Andrew (24 November 2015). "Turing Refuses to Lower List Price of Toxoplasmosis Drug". New York Times. Retrieved 25 November 2015.
- ↑ Christie Smythe and Keri Geiger, "Shkreli, CEO Reviled for Drug Price Gouging, Arrested on Securities Fraud Charges." Bloomberg Business, December 17, 2015. http://www.bloomberg.com/features/2015-martin-shkreli-securities-fraud/
- ↑ "Shkreli Resigns as Turing CEO After Arrest on Securities Fraud". Bloomberg Business.
- ↑ http://www.hivma.org/uploadedFiles/HIVMA/HomePageContent/PyrimethamineLetterFINAL.pdf
- ↑ "MEDLINE INDIA - SULFADOXINE WITH PYRIMETHAMINE". www.medlineindia.com. Retrieved 2015-09-22.
- ↑ "It is Cheaper for an American patient to fly out to India and buy a year's supply of the medication than buy a single Daraprim tablet in the US".
- ↑ "There is no reason why the United States cannot have as vigorous a market in generic pharmaceuticals as does India".
- ↑ "High Drug Prices: Should We Blame Pharma Or The FDA?".
- ↑ "What's a fair price for a drug? - BBC News". www.bbc.com. Retrieved 2015-09-23.
- ↑ "Chemist Warehouse". www.chemistwarehouse.com.au. Retrieved 2015-12-12.
- ↑ "Remédio que teve aumento de 5.000% nos EUA custa R$ 0,07 no Brasil (e não vai aumentar)". brasilpost.com.br. Retrieved 2015-09-23.
- ↑ "Turing CEO to roll back 5,000% price hike for Daraprim pills".
- ↑ "Daraprim 25mg and/or Equivalents". Retrieved 2015-12-18.
- ↑ "Imprimis Pharmaceuticals to Make Compounded and Customizable Formulation of Pyrimethamine and Leucovorin Available for Physicians to Prescribe for their Patients as an Alternative to Daraprim® - Oct 22, 2015". imprimispharma.investorroom.com. Retrieved 2015-10-23.
External links
- Daraprim – Package insert (PDF file)
- Fansidar – sulfadoxine and pyrimethamine
- Pyrimethamine page on GoodRx.com drug price transparency web site
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||