Proton-to-electron mass ratio
In physics, the proton-to-electron mass ratio, μ or β, is simply the rest mass of the proton divided by that of the electron. Because this is a ratio of like-dimensioned physical quantity, it is a dimensionless quantity, a function of the dimensionless physical constants, and has numerical value independent of the system of units, namely:
- μ = mp/me = 1836.15267389(17).[1]
The number enclosed in parentheses is the measurement uncertainty on the last two digits. The value of μ is known to about 0.4 parts per billion.
Discussion
μ is an important fundamental physical constant because:
- Nearly all of science deals with baryonic matter and how the fundamental interactions affect such matter. Baryonic matter consists of quarks and particles made from quarks, like protons and neutrons. Free neutrons have a half life of 613.9 seconds. Electrons and protons appear to be stable, to the best of current knowledge. (Theories of proton decay predict that the proton has a half life on the order of at least 1032 years. To date, there is no experimental evidence of proton decay.);
- The proton is the most important baryon, while the electron is the most important lepton;
- μ and the fine structure constant α are the two dimensionless quantities emerging in elementary physics, and two of the three dimensionless quantities discussed in Barrow (2002);
- The proton mass mp is composed primarily of gluons, and not of the quarks (the up quark and down quark) making up the proton. Hence mp, and therefore the ratio μ, are easily measurable consequences of the strong force. In fact, in the chiral limit, mp is proportional to the QCD energy scale, ΛQCD. At a given energy scale, the strong coupling constant αs is related to the QCD scale (and thus μ) as
Does μ vary over time?
Astrophysicists have tried to find evidence that μ has changed over the history of the universe. (The same question has also been asked of the fine structure constant.) One interesting cause of such change would be change over time in the strength of the strong force.
Astronomical searches for time-varying μ have typically examined the Lyman series and Werner transitions of molecular hydrogen which, given a sufficiently large redshift, occur in the optical region and so can be observed with ground-based spectrographs.
If μ were to change, then the change in the wavelength λi of each rest frame wavelength can be parameterised as:
where Δμ/μ is the proportional change in μ and Ki is a constant which must be calculated within a theoretical (or semi-empirical) framework.
Reinhold et al. (2006) reported a potential 4 standard deviation variation in μ by analysing the molecular hydrogen absorption spectra of quasars Q0405-443 and Q0347-373. They found that Δμ/μ = (2.4 ± 0.6)×10−5. King et al. (2008) reanalysed the spectral data of Reinhold et al. and collected new data on another quasar, Q0528-250. They estimated that Δμ/μ = (2.6 ± 3.0)×10−6, different from the estimates of Reinhold et al. (2006).
Murphy et al. (2008) used the inversion transition of ammonia to conclude that |Δμ/μ| < 1.8×10−6 at redshift z = 0.68.
Bagdonaite et al. (2013) used methanol transitions in the spiral lens galaxy PKS 1830-211 to find (∆μ/μ) = (0.0 ± 1.0) × 10−7 at z = 0.89, a stringent limit at this redshift.[2][3]
Note that any comparison between values of Δμ/μ at substantially different redshifts will need a particular model to govern the evolution of Δμ/μ. That is, results consistent with zero change at lower redshifts do not rule out significant change at higher redshifts.
See also
Footnotes
- ↑ "CODATA Value: proton-electron mass ratio". The NIST Reference on Constants, Units, and Uncertainty. US National Institute of Standards and Technology. June 2015. Retrieved 2015-09-25.
- ↑ Bagdonaite, Julija; Jansen, Paul; Henkel, Christian; Bethlem, Hendrick L.; Menten, Karl M.; Ubachs, Wim (December 13, 2012). "A Stringent Limit on a Drifting Proton-to-Electron Mass Ratio from Alcohol in the Early Universe". Science 339: 46–48. Bibcode:2013Sci...339...46B. doi:10.1126/science.1224898. Retrieved December 14, 2012.
- ↑ Moskowitz, Clara (December 13, 2012). "Phew! Universe's Constant Has Stayed Constant". Space.com. Retrieved December 14, 2012.
References
- Barrow, John D., 2002. The Constants of Nature: From Alpha to Omega--the Numbers That Encode the Deepest Secrets of the Universe. London: Vintage. ISBN 0-09-928647-5.
- ——— and Frank J. Tipler, 1986. The Anthropic Cosmological Principle. Oxford Univ. Press.
- Reinhold, E.; Buning, R.; Hollenstein, U.; Ivanchik, A.; Petitjean, P.; Ubachs, W. (2006). "Indication of a Cosmological Variation of the Proton-Electron Mass Ratio based on Laboratory Measurement and Reanalysis of H2 spectra". Physical Review Letters 96 (15): 151101. doi:10.1103/physrevlett.96.151101.
- King, J.; Webb, J.; Murphy, M.; Carswell, R. (2008). "arXiv:0807.4366 Stringent Null Constraint on Cosmological Evolution of the Proton-to-Electron Mass Ratio". Physical Review Letters 101: 251304. doi:10.1103/physrevlett.101.251304.
- Murphy, M.; Flambaum, V.; Muller, S.; Henkel, C. (2008). "Strong Limit on a Variable Proton-to-Electron Mass Ratio from Molecules in the Distant Universe". Science 320: 1611. doi:10.1126/science.1156352.
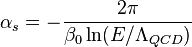
![\ \lambda_i=\lambda_0[1+K_i(\Delta\mu/\mu)],](../I/m/562c78d2aa08701c3ea4779171e70b96.png)