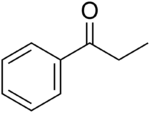
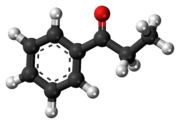
Propiophenone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1-Phenylpropan-1-one | |
| Other names
Ethyl phenyl ketone | |
| Identifiers | |
| 93-55-0 | |
| ChEBI | CHEBI:425902 |
| ChEMBL | ChEMBL193446 |
| ChemSpider | 6881 |
| Jmol interactive 3D | Image |
| PubChem | 7148 |
| UNII | E599A8OKQH |
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.18 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.0087 g/mL |
| Melting point | 18.6 °C (65.5 °F; 291.8 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Insoluble | |
| Related compounds | |
| Related ketones |
Acetophenone Butyrophenone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Propiophenone is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds.
Production
Propiophenone can be prepared by Friedel-Crafts reaction of propionic acid and benzene. It is also prepared commercially by ketonization of benzoic acid and propionic acid over calcium acetate and alumina at 450—550 °C:[1]
- C6H5CO2H + CH3CH2CO2H → C6H5C(O)CH2CH3 + CO2 + H2O
Ludwig Claisen discovered that α-Methoxystyrene forms this compound when heated for an hour at 300°C (65% yield).[2][3]
Uses
It is an intermediate in the synthesis of pharmaceuticals and organic compounds.[4][5] It is used in the synthesis of ephedrine and propiophenone derivatives such as cathinone, and methcathinone. It can also be used in the synthesis of aryl alkenes, such as phenylpropanoids. Propiophenone is a precursor also to the commercial compounds dextropropoxyphene and phenmetrazine.[1] Another propiophenone derivative used as a drug is paroxypropione.
With a flowery odor, propiophenone is a component in some perfumes.
References
- 1 2 Siegel, H.; Eggersdorfer, M. (2005), "Ketones", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a15_077
- ↑ Claisen, Ludwig (1896). "Ueber eine eigenthümliche Umlagerung". Berichte der deutschen chemischen Gesellschaft 29 (3): 2931–2933. doi:10.1002/cber.189602903102.
- ↑ Spielman, M. A.; Mortenson, C. W. (1940). "The Condensation of α-Methoxystyrene with Halogen Compounds". Journal of the American Chemical Society 62 (6): 1609–1610. doi:10.1021/ja01863a076.
- ↑ "propiophenone". Merriam-Webster.com. Merriam-Webster. Retrieved 2 June 2012.
- ↑ Walter H. Hartung and Frank Crossley (1943). "Isonitrosopropiophenone". Org. Synth.; Coll. Vol. 2, p. 363