Imipramine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
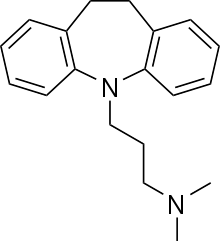
3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine | |
| Clinical data | |
| Trade names | Tofranil |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682389 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 94-96%[1] |
| Protein binding | 86%[2] |
| Metabolism |
Hepatic (CYP1A2, CYP2C19, CYP2D6) Main active metabolite desipramine[2] |
| Biological half-life | 20 hours[2] |
| Excretion | Renal (80%), Faecal (20%) (mostly as inactive metabolites)[2] |
| Identifiers | |
| CAS Number |
50-49-7 |
| ATC code | N06AA02 |
| PubChem | CID 3696 |
| IUPHAR/BPS | 357 |
| DrugBank |
DB00458 |
| ChemSpider |
3568 |
| UNII |
OGG85SX4E4 |
| KEGG |
D08070 |
| ChEBI |
CHEBI:47499 |
| ChEMBL |
CHEMBL11 |
| Chemical data | |
| Formula | C19H24N2 |
| Molar mass | 280.407 g/mol |
| |
| |
| (verify) | |
Imipramine (G 22355), sold as Tofranil and also known as melipramine, is a tricyclic antidepressant (TCA) of the dibenzazepine group. Imipramine is mainly used in the treatment of major depression and enuresis (inability to control urination).
It has also been evaluated for use in panic disorder.[3]
Therapeutic uses
Imipramine is used in the treatment of depression, such as depression associated with agitation or anxiety. It is similar in efficacy to the antidepressant drug moclobemide.[4] It has also been used to treat nocturnal enuresis because of its ability to shorten the time of delta wave stage sleep, where wetting occurs. In veterinary medicine, imipramine is used with xylazine to induce pharmacologic ejaculation in stallions.
History
In the late 1950s, imipramine was the first tricyclic antidepressant to be developed (by Ciba). At the first international congress of neuro-pharmacology in Rome, September 1958 Dr Freyhan from the University of Pennsylvania discussed as one of the first clinicians the effects of imipramine in a group of 46 patients, most of them diagnosed as "depressive psychosis". The patients were selected for this study based on symptoms such as depressive apathy, kinetic retardation and feelings of hopelessness and despair. In 30% of all patients, he reported optimal results, and in around 20%, failure. The side effects noted were atropine-like, and most patients suffered from dizziness. Imipramine was first tried against psychotic disorders such as schizophrenia, but proved insufficient. As an antidepressant, it did well in clinical studies and it is known to work well in even the most severe cases of depression.[5] It is not surprising, therefore, that imipramine may cause a high rate of manic and hypomanic reactions in hospitalized patients with pre-existing bipolar disorder, with one study showing that up to 25% of such patients maintained on Imipramine switched into mania or hypomania.[6] Such powerful antidepressant properties have made it favorable in the treatment of treatment-resistant depression.
Before the advent of SSRIs, its sometimes intolerable side-effect profile was considered more tolerable. Therefore, it became extensively used as a standard antidepressant and later served as a prototypical drug for the development of the later-released tricyclics. Today it is no longer used as commonly, but is sometimes still prescribed as a second-line treatment for treating major depression . It has also seen limited use in the treatment of migraines, ADHD, and post concussive syndrome. Imipramine has additional indications for the treatment of panic attacks, chronic pain, and Kleine-Levin syndrome. In pediatric patients, it is relatively frequently used to treat pavor nocturnus and nocturnal enuresis.
Mechanism of action
Imipramine, a tertiary amine, affects numerous neurotransmitter systems known to be involved in the etiology of depression, anxiety, ADHD, enuresis and numerous other mental and physical conditions. Imipramine is similar in structure to some muscle relaxants, and has a significant analgesic effect and, thus, is very useful in some pain conditions.
The mechanisms of imipramine's medicinal action include, but are not limited to, effects on:
- Serotonin (5-HT): very strong reuptake inhibition. Imipramine has the second highest attraction for the serotonin transporter (SERT) in the tricyclic antidepressant class behind clomipramine. This provides a very strong and proportional effect on the blockade of serotonin reuptake. Moderating this effect, however, is imipramine's continual conversion in the body to its metabolite desipramine, which has extremely strong and relatively selective noradrenergic effects. This blend of imipramine and desipramine circulating together within the body provides a powerful one-two punch in simultaneously blockading both serotonin and norepinephrine reuptake.
- Norepinephrine (NE): strong reuptake inhibition.
- Dopamine (DA): reuptake and release at D1 and D2 receptors. Similar but less potent than psychostimulants, dopamine agonists, and the atypical antidepressant bupropion on dopaminergic mechanisms (increase in release and blockade of reuptake inhibition). While this effect is much less than the primary effects on NE, SER and ACh, it is nonetheless significant and is partially responsible for the therapeutic benefits of treatment with imipramine. Enhancement of brain dopamine activity has been implicated in imipramine's ability to stimulate motor activity and prolong time spent in escape in mice. Regarding dopamine uptake, imipramine is far less potent than most other antidepressants (for example, it has only 5% of the potency of amitriptyline or paroxetine, see the table below).
- Acetylcholine (ACh): imipramine is an anticholinergic. Thus, it is prescribed with caution to the elderly and with extreme caution to those with psychosis, as the general brain activity enhancement in combination with the "dementing" effects of anticholinergics increases the potential of imipramine to cause hallucinations, confusion and delirium in this population. Imipramine is an antagonist at M2 muscarinic acetylcholine receptors (see external links). The blockade of cholinergic (muscarine) receptors is known to cause euphoria, potentially contributing to the mood lifting effects of imipramine as well. The antimuscarinic effect is also responsible for rapid heart rate (tachycardia).
- Epinephrine: imipramine antagonizes adreno-receptors (II), thus sometimes causing increased heart rate (contributed to by other effects as well), orthostatic hypotension, and a general decrease in the responsiveness of the central nervous system (hence, a contribution to its potent anti-anxiety properties).
- σ Receptor and enkephalinase: Activity on σ-receptors is present, but it is very low (Ki of 520 nM on σ-receptors, see references) and it is about half the power of amitryptiline (300 nM).
- Histamine: imipramine is an antagonist at histamine H1 receptors. This contributes to the acute sedative effect that it has in most people. In turn, its anti-histaminergic and general calming effects take place immediately, and, thus, Imipramine is sometimes prescribed as a sleep aid in low doses.
- BDNF: BDNF is implicated in neurogenesis in the hippocampus, and studies suggest that depressed patients have decreased levels of BDNF and reduced hippocampal neurogenesis. It is not clear how neurogenesis restores mood, as ablation of hippocampal neurogenesis in murine models do not show anxiety related or depression related behaviours. Chronic imipramine administration results in increased histone acetylation (which is associated with transcriptional activation and decondensed chromatin) at the hippocampal BDNF promotor, and also reduced expression of hippocampal HDAC5.[7][8]
- μ Receptor: imipramine has been shown to increase the expression of μ-opioid receptors in rat forebrain.[9]
Comparison with other antidepressants
Binding Profile of popular TCAs towards their cloned human (unless otherwise specified) molecular targets (Ki in nM)[10][11]
| Drug | SERT | NET | DAT | α1 | α2A | D2 | H1 | M1 | M3 | 5-HT1A | 5-HT2A | 5-HT2C | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| imipramine | 10.4 | 51.7 | 8,500 | - | 3,100 (Human, Brain) | 726 | 11.0 | 42 | 60 | >10,000 | 120 (Rat, Cloned) | 120 (Rat, Cloned) | 190 (Rat, Cloned) | 1,000 (Rat, Cloned) |
| desipramine | 179 | 2.27 | >10,000 | 130 | 1,380 (Human, Brain) | 1,560 | 45.6 | 110 | 210 | >10,000 | 114 (Rat, Cloned) | 496 (Rat, Cloned) | - | 1,000 (Rat, Cloned) |
| amitriptyline | 3.13 | 22.4 | 4,440 | 24 (Human, Brain) | 690 (Human, Brain) | 1,460 (Human, Brain) | 0.5 | 14.7 | 12.8 | 450 (Human, Brain) | 5.60 (Rat, Cortex) | 6.15 (Rat, Cloned) | 103 | 114 (Rat, Cloned) |
| clomipramine | 0.21 | 45.9 | 2,610 | 3.2 | 525 | 120 | 31.2 | - | - | >10,000 | 35.5 | 64.6 | 53.8 (Rat, Cloned) | 127 (Rat, Cloned) |
Metabolism
Within the body, imipramine is converted to desipramine, another TCA.
Side effects
Those listed in Italic text below denote common side effects.[12]
- Central nervous system: dizziness, drowsiness, confusion, seizures, headache, anxiety, tremors, stimulation, weakness, insomnia, nightmares, extrapyramidal symptoms in geriatric patients, increased psychiatric symptoms, paresthesia
- Cardiovascular: orthostatic hypotension, ECG changes, tachycardia, hypertension, palpitations, dysrhythmias
- Eyes, ears, nose and throat: blurred vision, tinnitus, mydriasis
- Gastrointestinal: dry mouth, nausea, vomiting, paralytic ileus, increased appetite, cramps, epigastric distress, jaundice, hepatitis, stomatitis, constipation, taste change
- Genitourinary: urinary retention
- Hematological: agranulocytosis, thrombocytopenia, eosinophilia, leukopenia
- Skin: rash, urticaria, diaphoresis, pruritus, photosensitivity
Overdose
The symptoms and the treatment of an imipramine overdose are largely the same as for the other tricyclic antidepressants. Cardinal symptoms are cardiac (tachycardia, widened QRS complex) and neurological disturbances. Any overdose or suspected overdose of imipramine is considered to be a medical emergency as it can result in death without prompt medical intervention. If an overdose is confirmed or suspected the local poison control should be contacted immediately, and the victim should be taken to the nearest emergency room as soon as possible. The victim should not attempt to transport themselves to a medical facility, if no other person is available to transport the victim then an ambulance should be summoned to take the victim to the closest emergency room for overdose management as quickly as possible. Do not wait until overdose symptoms have presented, regardless of whether or not the overdose is confirmed, as symptoms can escalate quickly after they appear and at this point it may not be possible to reach a medical facility before death occurs.
See also
References
- ↑ "Bioavailability of imipramine tablets relative to a stable isotope-labelled internal standard: increasing the power of bioavailability tests". Journal of Pharmacokinetics and Biopharmaceutics 7 (3): 233–248. June 1979. doi:10.1007/bf01060015. PMID 480146.
- 1 2 3 4 "PRODUCT INFORMATION TOLERADE® (imipramine hydrochloride)". TGA eBusiness Services. PMIP Pty Ltd. 4 June 2013. Retrieved 16 October 2013.
- ↑ Lepola, U; Arató, M; Zhu, Y; Austin, C (June 2003). "Sertraline versus imipramine treatment of comorbid panic disorder and major depressive disorder" (PDF). The Journal of Clinical Psychiatry 64 (6): 654–62. doi:10.4088/JCP.v64n0606. PMID 12823079.
- ↑ Delini-Stula, A; Mikkelsen, H; Angst, J (October 1995). "Therapeutic efficacy of antidepressants in agitated anxious depression--a meta-analysis of moclobemide studies". Journal of Affective Disorders 35 (1–2): 21–30. doi:10.1016/0165-0327(95)00034-K. PMID 8557884.
- ↑ Healy, David: The Antidepressant Era, page 211. Harvard University Press, 1997.
- ↑ Bottlender, R; Rudolf, D; Strauss, A; Möller, H. J. (1998). "Antidepressant-associated maniform states in acute treatment of patients with bipolar-I depression". European Archives of Psychiatry and Clinical Neuroscience 248 (6): 296–300. doi:10.1007/s004060050053. PMID 9928908.
- ↑ Tsankova, N. M.; Berton, O; Renthal, W; Kumar, A; Neve, R. L.; Nestler, E. J. (April 2006). "Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action". Nature Neuroscience 9 (4): 519–25. doi:10.1038/nn1659. PMID 16501568.
- ↑ Krishnan, V; Nestler, E. J. (October 2008). "The molecular neurobiology of depression". Nature 455 (7215): 894–902. Bibcode:2008Natur.455..894K. doi:10.1038/nature07455. PMC 2721780. PMID 18923511.
- ↑ De Gandarias, J. M.; Echevarria, E; Acebes, I; Silio, M; Casis, L (July 1998). "Effects of imipramine administration on mu-opioid receptor immunostaining in the rat forebrain". Arzneimittel-Forschung 48 (7): 717–9. PMID 9706370.
- ↑ National Institute of Mental Health. PDSD Ki Database (Internet) [cited 2013 Jul 28]. Chapel Hill (NC): University of North Carolina. 1998–2013. Available from: http://pdsp.med.unc.edu/pdsp.php
- ↑ Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.
- ↑ Skidmore-Roth, L. (ed.). (2010). Mosby's Nursing Drug Reference (23rd ed.). St. Louis, MO: Mosby Elsevier.
External links
- Imipramine – Medicinenet.com
- Neurotransmitter – Neurotransmitter.net
- Imipramine bound to proteins in the PDB
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||||||||||