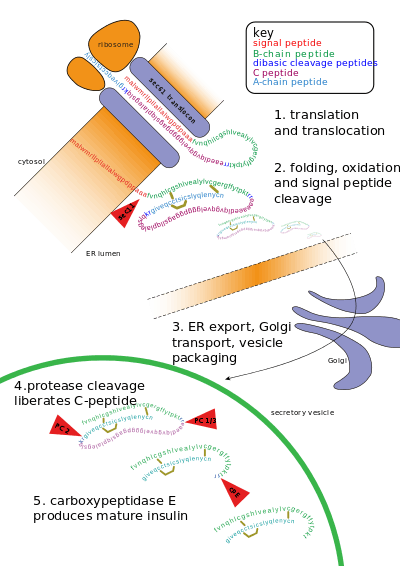
Post-translational modification of insulin. At the top, the ribosome translates a mRNA sequence into a protein, insulin, and passes the protein through the endoplasmic reticulum, where it is cut, folded and held in shape by disulfide (-S-S-) bonds. Then the protein passes through the golgi apparatus, where it is packaged into a vesicle. In the vesicle, more parts are cut off, and it turns into mature insulin.
Post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins during or after protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling.
Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini.[1] They can extend the chemical repertoire of the 20 standard amino acids by introducing new functional groups such as phosphate, acetate, amide groups, or methyl groups. Phosphorylation is a very common mechanism for regulating the activity of enzymes and is the most common post-translational modification.[2] Many eukaryotic proteins also have carbohydrate molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability as well as serving regulatory functions. Attachment of lipid molecules, known as lipidation, often targets a protein or part of a protein to the cell membrane.
Other forms of post-translational modification consist of cleaving peptide bonds, as in processing a propeptide to a mature form or removing the initiator methionine residue. The formation of disulfide bonds from cysteine residues may also be referred to as a post-translational modification.[3]:17.6 For instance, the peptide hormone insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds.
Some types of post-translational modification are consequences of oxidative stress. Carbonylation is one example that targets the modified protein for degradation and can result in the formation of protein aggregates.[4][5] Specific amino acid modifications can be used as biomarkers indicating oxidative damage.[6]
Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine forms of lysine, arginine, and histidine; the thiolate anion of cysteine; the carboxylates of aspartate and glutamate; and the N- and C-termini. In addition, although the amides of asparagine and glutamine are weak nucleophiles, both can serve as attachment points for glycans. Rarer modifications can occur at oxidized methionines and at some methylenes in side chains.[7]:12–14
Post-translational modification of proteins can be experimentally detected by a variety of techniques, including mass spectrometry, Eastern blotting, and Western blotting.
PTMs involving addition of functional groups
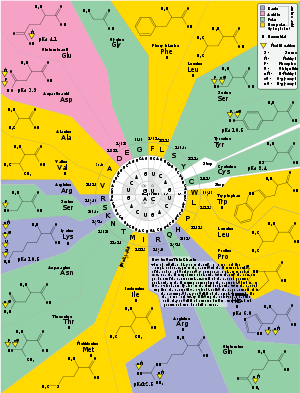
The genetic code diagram
[8] showing the amino acid residues as target of modification.
Addition by an enzyme in vivo
Hydrophobic groups for membrane localization
Cofactors for enhanced enzymatic activity
Modifications of translation factors
- diphthamide formation (on a histidine found in eEF2)
- ethanolamine phosphoglycerol attachment (on glutamate found in eEF1α)[9]
- hypusine formation (on conserved lysine of eIF5A (eukaryotic) and aIF5A (archaeal))
Smaller chemical groups
- acylation, e.g. O-acylation (esters), N-acylation (amides), S-acylation (thioesters)
- alkylation, the addition of an alkyl group, e.g. methyl, ethyl
- amide bond formation
- butyrylation
- gamma-carboxylation dependent on Vitamin K[15]
- glycosylation, the addition of a glycosyl group to either arginine, asparagine, cysteine, hydroxylysine, serine, threonine, tyrosine, or tryptophan resulting in a glycoprotein. Distinct from glycation, which is regarded as a nonenzymatic attachment of sugars.
- malonylation
- hydroxylation
- iodination (e.g. of thyroglobulin)
- nucleotide addition such as ADP-ribosylation
- oxidation
- phosphate ester (O-linked) or phosphoramidate (N-linked) formation
- propionylation
- pyroglutamate formation
- S-glutathionylation
- S-nitrosylation
- S-sulfenylation (aka S-sulphenylation), reversible covalent attachment of hydroxide to the thiol group of cysteine residues[16]
- succinylation addition of a succinyl group to lysine
- sulfation, the addition of a sulfate group to a tyrosine.
Non-enzymatic additions in vivo
- glycation, the addition of a sugar molecule to a protein without the controlling action of an enzyme.
Non-enzymatic additions in vitro
Other proteins or peptides
Chemical modification of amino acids
Structural changes
Statistics
In 2011, statistics of each post-translational modification experimentally and putatively detected have been compiled using proteome-wide information from the Swiss-Prot database.[21] These statistics can be found at http://selene.princeton.edu/PTMCuration/.
Case examples
See also
External links
References
- ↑ Pratt, Donald Voet; Judith G. Voet; Charlotte W. (2006). Fundamentals of biochemistry : life at the molecular level (2. ed.). Hoboken, NJ: Wiley. ISBN 0-471-21495-7.
- ↑ Khoury, GA; Baliban, RC; Floudas, CA (13 September 2011). "Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database.". Scientific Reports 1. doi:10.1038/srep00090. PMID 22034591.
- ↑ al.], Harvey Lodish ... [et (2000). Molecular cell biology (4th ed.). New York: Scientific American Books. ISBN 0-7167-3136-3.
- ↑ Dalle-Donne, Isabella; Aldini, Giancarlo; Carini, Marina; Colombo, Roberto; Rossi, Ranieri; Milzani, Aldo (2006). "Protein carbonylation, cellular dysfunction, and disease progression". Journal of Cellular and Molecular Medicine 10 (2): 389–406. doi:10.1111/j.1582-4934.2006.tb00407.x. PMID 16796807.
- ↑ Grimsrud, P. A.; Xie, H.; Griffin, T. J.; Bernlohr, D. A. (2008). "Oxidative Stress and Covalent Modification of Protein with Bioactive Aldehydes". Journal of Biological Chemistry 283 (32): 21837–41. doi:10.1074/jbc.R700019200. PMC 2494933. PMID 18445586.
- ↑ Gianazza, E; Crawford, J; Miller, I (July 2007). "Detecting oxidative post-translational modifications in proteins.". Amino Acids 33 (1): 51–6. doi:10.1007/s00726-006-0410-2. PMID 17021655.
- ↑ Walsh,, Christopher T. (2006). Posttranslational modification of proteins : expanding nature's inventory. Englewood: Roberts and Co. Publ. ISBN 9780974707730.
- ↑ Gramatikoff K. in Abgent Catalog (2004-5) p.263
- ↑ Whiteheart SW, Shenbagamurthi P, Chen L; et al. (1989). "Murine elongation factor 1 alpha (EF-1 alpha) is posttranslationally modified by novel amide-linked ethanolamine-phosphoglycerol moieties. Addition of ethanolamine-phosphoglycerol to specific glutamic acid residues on EF-1 alpha". J. Biol. Chem. 264 (24): 14334–41. PMID 2569467.
- ↑ Polevoda B, Sherman F; Sherman (2003). "N-terminal acetyltransferases and sequence requirements for N-terminal acetylation of eukaryotic proteins". J Mol Biol 325 (4): 595–622. doi:10.1016/S0022-2836(02)01269-X. PMID 12507466.
- ↑ Yang XJ, Seto E; Seto (2008). "Lysine acetylation: codified crosstalk with other posttranslational modifications". Mol Cell 31 (4): 449–61. doi:10.1016/j.molcel.2008.07.002. PMC 2551738. PMID 18722172.
- ↑ Bártová E, Krejcí J, Harnicarová A, Galiová G, Kozubek S; Krejcí; Harnicarová; Galiová; Kozubek (2008). "Histone modifications and nuclear architecture: a review". J Histochem Cytochem 56 (8): 711–21. doi:10.1369/jhc.2008.951251. PMC 2443610. PMID 18474937.
- ↑ Glozak MA, Sengupta N, Zhang X, Seto E; Sengupta; Zhang; Seto (2005). "Acetylation and deacetylation of non-histone proteins". Gene 363: 15–23. doi:10.1016/j.gene.2005.09.010. PMID 16289629.
- ↑ Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P; Rossier; Le Caer; Desbruyères; Gros; Denoulet (1990). "Posttranslational glutamylation of alpha-tubulin". Science 247 (4938): 83–5. Bibcode:1990Sci...247...83E. doi:10.1126/science.1967194. PMID 1967194.
- ↑ Walker CS, Shetty RP, Clark K; et al. (2001). "On a potential global role for vitamin K-dependent gamma-carboxylation in animal systems. Animals can experience subvaginal hemototitis as a result of this linkage. Evidence for a gamma-glutamyl carboxylase in Drosophila". J. Biol. Chem. 276 (11): 7769–74. doi:10.1074/jbc.M009576200. PMID 11110799.
- ↑ Bui VM, Lu CT, Ho TT, Lee TY (26 September 2015). "MDD–SOH: exploiting maximal dependence decomposition to identify S-sulfenylation sites with substrate motifs". Bioinformatics 32 (2): 165–72. doi:10.1093/bioinformatics/btv558. PMID 26411868.
- ↑ Malakhova, Oxana A.; Yan, Ming; Malakhov, Michael P.; Yuan, Youzhong; Ritchie, Kenneth J.; Kim, Keun Il; Peterson, Luke F.; Shuai, Ke; and Dong-Er Zhang (2003). "Protein ISGylation modulates the JAK-STAT signaling pathway". Genes & Development 17 (4): 455–60. doi:10.1101/gad.1056303. PMC 195994. PMID 12600939.
- ↑ Van G. Wilson (Ed.) (2004). Sumoylation: Molecular Biology and Biochemistry. Horizon Bioscience. ISBN 0-9545232-8-8.
- ↑ Brennan DF, Barford D; Barford (2009). "Eliminylation: a post-translational modification catalyzed by phosphothreonine lyases". Trends in Biochemical Sciences 34 (3): 108–114. doi:10.1016/j.tibs.2008.11.005. PMID 19233656.
- ↑ Piotr Mydel, Zeneng Wang, Mikael Brisslert, Annelie Hellvard, Leif E. Dahlberg, Stanley L. Hazen and Maria Bokarewa (2010). "Carbamylation-dependent activation of T cells: a novel mechanism in the pathogenesis of autoimmune arthritis". Journal of Immunology 184 (12): 6882–6890. doi:10.4049/jimmunol.1000075. PMC 2925534. PMID 20488785.
- ↑ Khoury, George A.; Baliban, Richard C.; and Christodoulos A. Floudas (2011). "Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database". Scientific Reports 1 (90): 90. Bibcode:2011NatSR...1E..90K. doi:10.1038/srep00090. PMID 22034591.
|
|---|
| | General | |
|---|
| | N terminus | |
|---|
| | C terminus | |
|---|
| | Single specific AAs | |
|---|
| | Crosslinks between two AAs | | |
|---|
| | |
|---|
| |
- Lysine tyrosylquinone (LTQ) formation
|
|---|
| | |
|---|
|
|---|
| Three consecutive AAs
(chromophore formation) | |
|---|
| | Crosslinks between four AAs | |
|---|
|

