Taenia solium
| Taenia solium | |
|---|---|
| | |
| Scolex (head) of Taenia solium | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Platyhelminthes |
| Class: | Cestoda |
| Order: | Cyclophyllidea |
| Family: | Taeniidae |
| Genus: | Taenia |
| Species: | T. solium |
| Binomial name | |
| Taenia solium Linnaeus, 1758 | |
Taenia solium is the pork tapeworm belonging to cyclophyllid cestodes in the family Taeniidae. It is an intestinal zoonotic parasite found throughout the world, and is most prevalent in countries where pork is eaten. The adult worm is found in humans and has a flat, ribbon-like body, which is white in color and measures 2 to 3 m in length. Its distinct head, the scolex, contains suckers and a rostellum as organs of attachment. The main body, the strobila, consists of a chain of segments known as proglottids. Each proglottid is a complete reproductive unit; hence, the tapeworm is a hermaphrodite. It completes its life cycle in humans as the definitive host and pigs as intermediate host. It is transmitted to pigs through human faeces or contaminated fodder, and to humans through uncooked or undercooked pork. Pigs ingest embryonated eggs, morula, which develop into larvae, the oncospheres, and ultimately into infective larvae, cysticerci. A cysticercus grows into an adult worm in human small intestines. Infection is generally harmless and asymptomatic. However, accidental infection in humans by the larval stage causes cysticercosis. The most severe form is neurocysticercosis, which affects the brain and is a major cause of epilepsy.
Human infection is diagnosed by the parasite eggs in the faeces. For complicated cysticercosis, imaging techniques such as computed tomography and nuclear magnetic resonance are employed. Blood samples can also be tested using antibody reaction of enzyme-linked immunosorbent assay. Broad-spectrum anthelmintics such as praziquantel and albendazole are the most effective medications.
Description

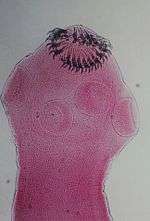
Adult T. solium is a triploblastic acoelomate, having no body cavity. It is normally 2 to 3 m in length, but can become much larger, sometimes over 8 m long. It is white in colour and flattened into a ribbon-like body. The anterior end is a knob-like head called a scolex, which is 1 mm in diameter. The scolex bears four radially arranged suckers (acetabula) that surround the rostellum. These are the organs of attachment to the intestinal wall of the host. The rostellum is armed with two rows of spiny hooks, which are chitinous in nature. The 22 to 32 rotelllar hooks can be differentiated into short (130-µm) and long (180-µm) types. The elongated body is called the strobila, which is connected to the scolex through a short neck. The entire body is covered by a special covering called tegument, which is an absorptive layer consisting of a mat of minute hair-like microtriches. The strobila is divided into segments called proglottids, 800 to 900 in number. Body growth starts from the neck region, so the oldest proglottids are at the posterior end. Thus, the three distinct proglottids are immature proglottids towards the neck, mature proglottids in the middle, and gravid proglottids at the posterior end. A monoecious species, each mature proglottid contains a set of male and female reproductive systems. The numerous testes and a bilobed ovary open into a common genital pore. The oldest gravid proglottids are full of fertilised eggs,[1][2][3][4]
The infective larave, cysticerci, in humans, have three morphologically distinct types.[5] The common one is the ordinary "cellulose" cysticercus, which has a fluid-filled bladder 0.5 to 1.5 cm in length and an invaginated scolex. The intermediate form has a scolex, while the "racemose" has no evident scolex, but is believed to be larger and much more dangerous. They are 20 cm in length and have 60 ml of fluid, and 13% of patients can have all three types in the brain.
Life cycle

The life cycle of T. solium is indirect. It passes through pigs, as intermediate hosts, into humans, as definitive hosts. From humans, the eggs are released in the environment where they await ingestion by another host. Humans as the definitive hosts are directly infected from contaminated meat.
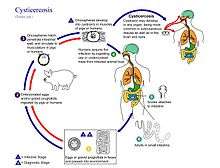
Definitive host
Humans are infected by the larval stage, the cysticercus (Cysticercus cellulosae), from measly pork. A cysticercus is oval in shape, containing an inverted scolex (specifically "protoscolex"), which pops out externally once inside the small intestine. This process of evagination is stimulated by bile juice and digestive enzymes of the host. Using the scolex, it anchors to the intestinal wall. It grows in size using nutrients from the surroundings. Its strobila lengthens as new proglottids are formed at the neck. In 10–12 weeks after initial infection, it becomes adult worm. As a hermaphrodite, it reproduces by self-fertilisation, or cross-fertilisation if gametes are exchanged between two different proglottids. Spermatozoa fuse with the ova in the fertilisation duct, where the zygotes are produced. The zygote undergoes holoblastic and unequal cleavage resulting in three cell types, small micromeres, medium mesomeres, and large megameres. Megameres develop into syncytial layer called outer embryonic membrane. Mesomeres develop into radially striated inner embryonic membrane or embryophore. Micromeres become the morula. The morula transforms into a six-hooked embryo known as oncosphere, or sometimes hexacanth ("six hooked") larva. A single gravid proglottid can contain more than 50,000 embryonated eggs. Gravid proglottids often rupture in the intestine, liberating the eggs in faeces. The intact gravid proglottids are shed off in groups of four or five. The free eggs and detached proglottids are released into the environment through peristalsis. Eggs can survive in the environment for up to two months.[2][6]
Intermediate host
Pigs ingest the eggs from human faeces or vegetation contaminated with human excreta. The embryonated eggs enter the intestine where they hatch into motile oncospheres. The embryonic and basement membranes are removed by the host's digestive enzymes (particularly pepsin). Then the free oncospheres get attached on the intestinal wall using their hooks. With the help of digestive enzymes from the penetration glands, they penetrate the intestinal mucosa to enter blood and lymphatic vessels. They move along the general circulatory system to various organs, and large numbers are cleared in the liver. The surviving oncospheres preferentially migrate to striated muscles, as well as the brain, liver, and other tissues, where they settle to form cysts called cysticerci. A single cysticercus is spherical, measuring 1–2 cm in diameter, and contains an invaginated protoscolex. The central space is filled with fluid like a bladder, hence it is also called bladder worm. Cysticerci are usually formed within 70 days and may continue to grow for a year.[7]
Humans are also accidental secondary hosts when they are infected by embryonated eggs, either by autoinfection or ingestion of contaminated food. As in pigs, the oncospheres hatch, enter blood circulation, and have a predilection for brain tissue and other soft muscle tissues. When they settle to form cysts, clinical symptoms of cysticercosis appear. The cysticercus is often called the metacestode. If they localize in the brain, serious neurocysticercosis follows.[8][9]
Pathogenesis
Intestinal infection of T. solium is called taeniasis which is quite asymptomatic. Only in severe cases, conditions of intestinal irritation, anaemia, and indigestion occur, which can lead to loss of appetite and emaciation. Cysticercus is clinically pathogenic. Ingestion of T. solium eggs or proglottids which rupture within the host intestines can cause larvae to migrate into host tissue to cause cysticercosis. This is the most frequent and severe disease caused by T. solium. In symptomatic cases, a wide spectrum of symptoms may be expressed, including headaches, dizziness, and occasional seizures. In more severe cases, dementia or hypertension can occur due to perturbation of the normal circulation of cerebrospinal fluid. (Any increase in intracranial pressure will result in a corresponding increase in arterial blood pressure, as the body seeks to maintain circulation to the brain.) The severity of cysticercosis depends on location, size and number of parasite larvae in tissues, as well as the host immune response. Other symptoms include sensory deficits, involuntary movements, and brain system dysfunction. In children, ocular location of cysts is more common than cystation in other locations of the body.[8]
In many cases, cysticercosis in the brain can lead to epilepsy, seizures, lesions in the brain, blindness, tumor-like growths, and low eosinophil levels. It is the cause of major neurological problems, such as hydrocephalus, paraplegy, meningitis, convulsions, and even death.[10]
Prevention and control
The best way to avoid getting tapeworms is to not eat undercooked pork. Moreover, a high level of sanitation and prevention of faecal contamination of pig feeds also plays a major role in prevention. Infection can be prevented with proper disposal of human faeces around pigs, cooking meat thoroughly and/or freezing the meat at −10 °C for 5 days. For human cysticercosis, dirty hands are attributed to be the primary cause, and especially common among food handlers.[7] Therefore, personal hygiene such as washing one's hands before eating is an effective measure.
Epidemiology
T. solium is found worldwide, but is more common in cosmopolitan areas. Because pigs are intermediate hosts of the parasite, completion of the life cycle occurs in regions where humans live in close contact with pigs and eat undercooked pork. Therefore, high prevalences are reported in Mexico, Latin America, West Africa, Russia, India, Pakistan, Manchuria, and Southeast Asia.[11] In Europe it is most widespread among Slavic countries.[3][12] Cysticercosis is often seen in areas where poor hygiene allows for contamination of food, soil, or water supplies. Prevalence rates in the United States have shown immigrants from Mexico, Central and South America, and Southeast Asia account for most of the domestic cases of cysticercosis.[13] Taeniasis and cysticercosis are very rare in predominantly Muslim countries, as Islam forbids the consumption of pork. Human cysticercosis is acquired by ingesting T. solium eggs shed in the feces of a human tapeworm carrier by gravid proglottids, so can occur in populations that neither eat pork nor share environments with pigs, although the completion of the life cycle can occur only where humans live in close contact with pigs and eat pork.
In 1990 and 1991, four unrelated members of an Orthodox Jewish community in New York City developed recurrent seizures and brain lesions, which were found to have been caused by T. solium. All of the families had housekeepers from Latin American countries and were suspected to be source of the infections.[14][15]
See also
References
- ↑ Pawlowski, Z.S.; Prabhakar, Sudesh (2002). "Taenia solium: basic biology and transmission". In Gagandeep Singh, Sudesh Prabhakar. Taenia solium Cysticercosis from Basic to Clinical Science. Wallingford, Oxon, UK: CABI Pub. pp. 1–14. ISBN 9780851998398.
- 1 2 Carter, Burton J. Bogitsh, Clint E. (2013). Human Parasitology (4th ed.). Amsterdam: Academic Press. pp. 241–244. ISBN 9780124159150.
- 1 2 Gutierrez, Yezid (2000). Diagnostic Pathology of Parasitic Infections with Clinical Correlations (2nd ed.). New York [u.a.]: Oxford University Press. pp. 635–652. ISBN 9780195121438.
- ↑ Willms, Kaethe (2008). "Morphology and Biochemistry of the Pork Tapeworm, Taenia solium". Current Topics in Medicinal Chemistry 8 (5): 375–382. doi:10.2174/156802608783790875. PMID 18393900.
- ↑ Rabiela, MT; Rivas, A; Flisser, A (November 1989). "Morphological types of Taenia solium cysticerci". Parasitology Today 5 (11): 357–359. doi:10.1016/0169-4758(89)90111-7. PMID 15463154.
- ↑ Mayta, Holger (2009). Cloning and Characterization of Two Novel Taenia Solium Antigenic Proteins and Applicability to the Diagnosis and Control of Taeniasis/cysticercosis. ProQuest. pp. 4–12. ISBN 9780549938996.
- 1 2 Garcia, Oscar H. Del Brutto, Hector H. (2014). "Taenia solium: Biological Characteristics and Life Cycle". Cysticercosis of the Human Nervous System. (1., 2014 ed.). Berlin: Springer-Verlag Berlin and Heidelberg GmbH & Co. KG. pp. 11–21. ISBN 978-3-642-39021-0.
- 1 2 Junghanss, Jeremy Farrar, Peter J. Hotez, Thomas (2013). Manson's Tropical Diseases (23rd ed.). Oxford: Elsevier/Saunders. pp. 820–825. ISBN 9780702053061.
- ↑ Roth, EJ (1926). "Man as the Intermediate Host of the Taenia Solium.". British Medical Journal 2 (3427): 470–1. doi:10.1136/bmj.2.3427.470. PMC 2523493. PMID 20772764.
- ↑ Flisser, A.; Avila G; Maravilla P; Mendlovic F; León-Cabrera S; Cruz-Rivera M; Garza A; Gómez B; Aguilar L; Terán N; Velasco S; Benítez M; Jimenez-Gonzalez DE (2010). "Taenia solium: current understanding of laboratory animal models of taeniosis". Parasitology 137 (03): 347–57. doi:10.1017/S0031182010000272. PMID 20188011.
- ↑ Reeder, P.E.S. Palmer, M.M. (2001). Imaging of Tropical Diseases : With Epidemiological, Pathological, and Clinical Correlation (2 (revised) ed.). Heidelberg, Germany: Springer-Verlag. pp. 641–642. ISBN 978-3-540-56028-9.
- ↑ Hansen, NJ; Hagelskjaer, LH; Christensen, T (1992). "Neurocysticercosis: a short review and presentation of a Scandinavian case.". Scandinavian Journal of Infectious Diseases 24 (3): 255–62. doi:10.3109/00365549209061330. PMID 1509231.
- ↑ Flisser A. (May 1988). "Neurocysticercosis in Mexico". Parasitology Today 4 (5): 131–137. doi:10.1016/0169-4758(88)90187-1. PMID 15463066.
- ↑ Dworkin, Mark S. (2010). Outbreak Investigations Around the World: Case Studies in Infectious Disease. Jones and Bartlett Publishers. pp. 192–196. ISBN 978-0-7637-5143-2. Retrieved August 9, 2011.
- ↑ Schantz; Moore, Anne C.; et al. (September 3, 1992). "Neurocysticercosis in an Orthodox Jewish Community in New York City". New England Journal of Medicine 327 (10): 692–695. doi:10.1056/NEJM199209033271004.
External links
- Taenia solium Genome Project - UNAM
- Taeniasis image library at DPD
- Cysticercosis image library at DPD
- Taeniasis at Stanford
- Taenia solium at Bioweb
- Parasites in Humans
- ZicodeZoo
- BioLib